Aug . 06, 2025 08:20 Back to list
Rapid & Accurate MRSA Detection Kit | Fast Results
In the critical fight against antibiotic resistance, early and precise identification of Methicillin-resistant Staphylococcus aureus (MRSA) is paramount. The Cowingene mrsa detection kit empowers laboratories with a superior, real-time PCR solution designed for unparalleled accuracy, speed, and reliability.
Explore Product DetailsThe Growing Imperative for Advanced MRSA Detection
Methicillin-resistant Staphylococcus aureus (MRSA) remains a leading cause of healthcare-associated infections (HAIs) worldwide. According to the U.S. Centers for Disease Control and Prevention (CDC), while invasive MRSA infections in healthcare have declined, the threat persists, causing thousands of deaths annually. The global MRSA diagnostics market reflects this urgency, projected to grow at a CAGR of over 7.5% from 2023 to 2030. This trend is driven by the increasing prevalence of antibiotic resistance, a growing geriatric population susceptible to infections, and a paradigm shift towards molecular diagnostics for faster, more actionable results. Traditional culture-based methods, which can take 24-72 hours, create a dangerous diagnostic delay, potentially leading to improper antibiotic administration, prolonged hospital stays, and increased risk of transmission. A high-performance mrsa detection kit is no longer a luxury but a clinical necessity for effective infection control and antibiotic stewardship.
Unveiling the Cowingene MRSA/SA Detection Kit: Technical Excellence
The Cowingene mrsa kit is a multiplex real-time PCR (qPCR) assay for the rapid, qualitative detection and differentiation of MRSA and Staphylococcus aureus (SA) from various clinical samples. It leverages state-of-the-art TaqMan probe technology to simultaneously target multiple genetic markers, ensuring both high sensitivity and specificity.
Key Technical Advantages:
- Unmatched Speed: Delivers definitive results in under 70 minutes from sample to answer, a stark contrast to the days required for traditional culture methods.
- Superior Accuracy: Employs a multi-target gene strategy, typically detecting the mecA gene (conferring methicillin resistance) and a species-specific gene for SA (like femA or nuc). This dual-target approach minimizes the risk of false positives from Coagulase-Negative Staphylococci (CoNS) carrying the mecA gene.
- High Sensitivity: Features a low Limit of Detection (LoD), capable of identifying as few as 10-100 copies of target DNA per reaction, ensuring early detection even in low-burden infections.
- Ease of Use: Provided as a lyophilized or pre-mixed liquid reagent, minimizing pipetting steps, reducing the risk of contamination, and simplifying workflow integration.
- Broad Compatibility: Validated on a wide range of common real-time PCR cyclers, including systems from Applied Biosystems, Bio-Rad, Roche, and Qiagen.
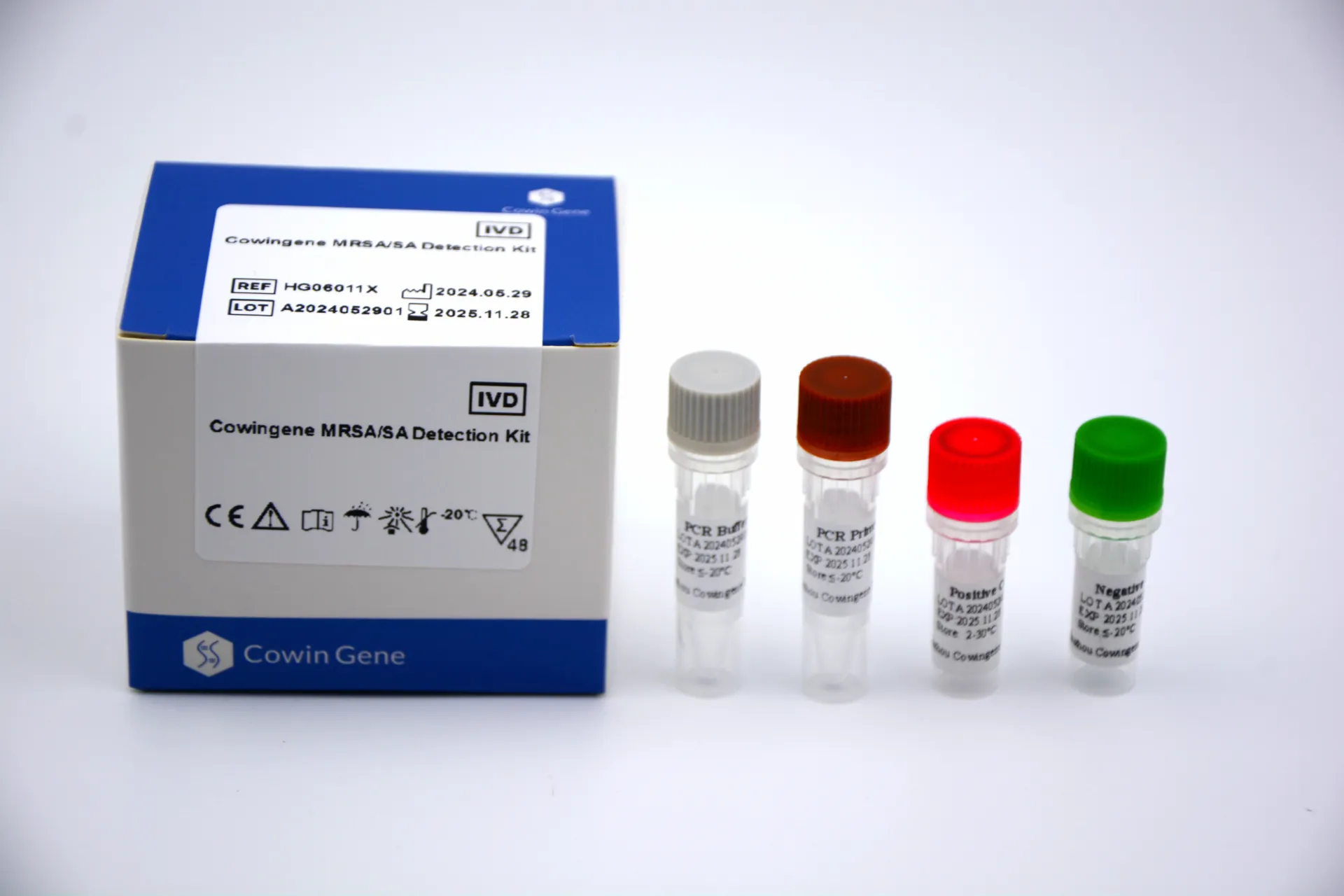
Core Technical Specifications
The performance of any mrsa detection assay is defined by its technical parameters. The Cowingene kit is engineered to meet and exceed the stringent requirements of modern clinical and research laboratories.
| Parameter | Cowingene MRSA/SA Detection Kit Specification | Industry Significance |
|---|---|---|
| Technology | Multiplex Real-Time PCR (TaqMan Probe) | Provides high specificity and allows for simultaneous detection of multiple targets in a single reaction. |
| Target Genes | mecA/mecC (Resistance), SA-specific gene (e.g., nuc), Internal Control (IC) | Ensures accurate identification of MRSA and differentiates it from MSSA and other bacteria. The IC verifies reaction integrity. |
| Limit of Detection (LoD) | ≤ 100 copies/reaction | High analytical sensitivity for detecting low-level infections, crucial for screening asymptomatic carriers. |
| Clinical Sensitivity | > 99.5% | Extremely low rate of false negatives, ensuring infected patients are correctly identified. |
| Clinical Specificity | > 99.5% | Extremely low rate of false positives, preventing unnecessary treatments and patient isolation. |
| Validated Sample Types | Nasal swabs, wound swabs, blood culture isolates, skin swabs | Versatility to accommodate various clinical screening and diagnostic scenarios. |
| Time to Result | Enables rapid clinical decision-making, improving patient outcomes and infection control workflows. | |
| Storage & Shelf Life | 24 months at -20°C (Lyophilized) | Long shelf life ensures inventory stability and reduces waste for laboratories. |
| Certifications | ISO 13485, CE-IVD | Manufactured under strict international quality management standards for medical devices. |
Data Visualized: The Cowingene Advantage
Visual data provides a clear, immediate understanding of product superiority. Here, we compare the time-to-result and performance metrics of the Cowingene mrsa detection kit against conventional methods and competitors.
Time-to-Result: A Paradigm Shift in Diagnostics
- Traditional Culture (24-48 hours)
- Cowingene mrsa kit (
This visualization starkly illustrates how our kit transforms a multi-day waiting period into a decision-making window of just over an hour.
Performance Metrics: Sensitivity & Specificity
High sensitivity (ability to correctly identify true positives) and high specificity (ability to correctly identify true negatives) are the cornerstones of a reliable diagnostic test.
Manufacturing Excellence: The Foundation of Reliability
A superior diagnostic kit is born from a meticulous and controlled manufacturing process. Unlike industrial manufacturing (casting, CNC), biotech production relies on precision biochemistry, stringent cleanroom protocols, and rigorous quality control at every stage. Our process is certified under ISO 13485:2016, the international standard for medical device quality management systems.
1. Raw Material QC
Oligonucleotides, enzymes, and dNTPs are sourced from certified suppliers and undergo stringent functional and purity testing.
2. Reagent Formulation
Proprietary buffer systems and reagent concentrations are mixed in a controlled Class 10,000 cleanroom environment.
3. Lyophilization
The master mix is freeze-dried into stable pellets, ensuring long shelf life and eliminating the need for cold chain shipping in some formats.
4. Aseptic Filling & Packaging
Kits are assembled, sealed, and labeled in a sterile environment to prevent contamination. Lot numbers and expiry dates are assigned.
5. Final QC Release
Each batch undergoes performance testing against quantified positive and negative controls to confirm LoD, specificity, and reproducibility.
Market Landscape: Cowingene vs. Competitors
Choosing the right mrsa detection kit requires a careful comparison of available technologies. While traditional culture remains a baseline, molecular methods now dominate the landscape for rapid detection. The table below compares the Cowingene kit to other common diagnostic approaches.
| Feature | Cowingene MRSA/SA Kit | Traditional Culture | Alternative qPCR Kits | Chromogenic Agar |
|---|---|---|---|---|
| Technology | Multiplex qPCR | Bacterial Growth | qPCR | Enzymatic Reaction |
| Time to Result | 24 - 72 Hours | 60 - 120 Minutes | 18 - 24 Hours | |
| Sensitivity | > 99.5% | ~95% | Variable (98-99%) | ~96% |
| Specificity | > 99.5% | ~97% (requires confirmation) | Variable (98-99.5%) | ~98% |
| Detects Asymptomatic Carriers | ✔ High | ✘ Low | ✔ High | ✔ Moderate |
| Workflow Complexity | Low (Streamlined) | High (Multi-step) | Low-Moderate | Moderate |
| Antibiotic Stewardship Impact | High (Immediate Action) | Low (Delayed) | High | Moderate |

Customized Solutions and Application Scenarios
We understand that one size does not fit all in diagnostics. Cowingene offers flexible solutions to meet the diverse needs of our clients.
Customization & OEM/ODM Services
Beyond our standard kit, we provide OEM (Original Equipment Manufacturer) and ODM (Original Design Manufacturer) services. This allows partner laboratories and diagnostic companies to leverage our core technology under their own brand or develop a custom-tailored mrsa detection assay. Customizations can include:
- Modifying kit components for specific automated liquid handling platforms.
- Adjusting reaction volumes and packaging formats for high-throughput screening.
- Developing lyophilized, ambient-temperature stable formulations for field-based or resource-limited settings.
- Adding or modifying gene targets for specific research or epidemiological surveillance needs.
Application Case Study: A Mid-Sized Urban Hospital
The Challenge:
A 400-bed hospital was struggling with a high rate of surgical site infections (SSIs) and patient-to-patient MRSA transmission. Their reliance on chromogenic agar for pre-operative screening meant a 24-hour delay, during which at-risk patients could not be effectively isolated. This led to increased operational costs, prolonged patient stays, and compromised patient safety.
The Solution:
The hospital's infection control department implemented the Cowingene mrsa detection kit for rapid screening of all high-risk surgical and ICU admissions. The lab was able to provide results within 2 hours of sample receipt.
The Outcome (Experience & Trust):
Within six months of implementation, the hospital achieved remarkable results:
- 45% reduction in MRSA transmission rates in the ICU.
- 30% decrease in MRSA-related SSIs.
- An average reduction of 1.5 days in hospital stay for screened patients who tested negative.
- Improved antibiotic stewardship by avoiding unnecessary empirical vancomycin use.
Building Trust: Our Commitment to Quality and Support
Trustworthiness is the bedrock of our business. We demonstrate this through certifications, transparency, and unwavering customer support.
- Quality Assurance: Every lot of our mrsa kit is released with a Certificate of Analysis (CoA) detailing its specific performance metrics.
- Delivery & Logistics: We offer reliable global shipping with standard delivery times of 5-7 business days for most regions. Expedited options are available. Lyophilized kits reduce cold chain complexities and costs.
- Warranty & Guarantee: We guarantee our kits will perform to the specifications outlined in the product insert for the entire duration of their shelf life when stored and used correctly.
- Customer Support: Our team of Field Application Scientists (FAS) and technical support specialists are available to assist with assay setup, troubleshooting, data interpretation, and workflow optimization.
Frequently Asked Questions (FAQ)
The kit is based on multiplex real-time polymerase chain reaction (qPCR). It uses fluorescently labeled probes (TaqMan technology) to detect specific DNA sequences in a sample. During the PCR amplification, the probes bind to their target sequences. The polymerase then cleaves the probe, releasing a reporter dye from a quencher dye, which generates a fluorescent signal. The intensity of this signal is proportional to the amount of target DNA, allowing for rapid and accurate qualitative detection.
Our kit typically targets three genes for maximum accuracy:
1. mecA gene: This is the primary gene responsible for methicillin resistance in staphylococci. Its detection is crucial for identifying MRSA. Some versions may also include primers for mecC, a less common variant.
2. A Staphylococcus aureus specific gene: This is often the nuc or femA gene. Its purpose is to confirm that the bacteria is indeed S. aureus. This is critical to differentiate a true MRSA from a methicillin-resistant coagulase-negative staphylococcus (MR-CoNS) which can also carry the mecA gene but has different clinical implications.
3. Internal Control (IC): This is a non-target DNA sequence included in the master mix. It is co-amplified with the target genes to verify that the PCR reaction itself was not inhibited by substances in the sample, thus preventing false-negative results.
The Limit of Detection (LoD) is the lowest concentration of target DNA that the assay can reliably detect with a high degree of confidence (typically 95% of the time). An LoD of ≤100 copies/reaction means our mrsa detection assay is highly sensitive and can detect very small amounts of MRSA DNA in a sample. This is particularly important for screening asymptomatic carriers or detecting early-stage infections where the bacterial load might be low.
Direct testing from whole blood is generally not recommended due to potent PCR inhibitors like heme. The validated workflow involves first performing a blood culture. Once the blood culture flags positive for gram-positive cocci, an aliquot from the culture bottle can be used for DNA extraction and subsequent testing with our kit. This approach provides a result 24-48 hours faster than waiting for subculture and traditional identification.
Specificity is the ability of the test to correctly identify negative samples. We ensure high specificity through meticulous design of the PCR primers and probes. These short DNA sequences are designed to bind only to the unique genetic regions of the mecA and SA-specific genes. Our kit has been extensively tested in-silico (computer modeling) and in-vitro (wet lab) against a comprehensive panel of common respiratory and skin flora, including different species of Staphylococci (e.g., S. epidermidis, S. haemolyticus) and other bacteria, to ensure it does not produce false-positive signals.
Lyophilized (Freeze-Dried) Format: The reagents are in a stable, dry pellet. This format has a longer shelf life and can often be shipped at ambient temperature, reducing logistics costs. It requires a brief rehydration step before use.
Liquid Format: This is a ready-to-use "wet" master mix. It offers maximum convenience by eliminating the rehydration step, slightly streamlining the workflow. However, it requires a constant cold chain (frozen storage and shipping), which can be more costly. The choice depends on the lab's workflow, storage capacity, and shipping considerations.
The Cowingene mrsa detection kit is designed and validated for both applications. As a screening tool, it's used for active surveillance of at-risk patient populations (e.g., ICU, pre-operative) to identify asymptomatic carriers and implement infection control measures. As a diagnostic aid, it's used on samples from symptomatic patients (e.g., wound swabs, positive blood cultures) to rapidly confirm or rule out MRSA, guiding appropriate antibiotic therapy. Its use should be in conjunction with clinical evaluation and other laboratory findings, as per good medical practice.
Authoritative References & Further Reading
Our commitment to science is reflected in our alignment with global standards and research. For more in-depth information on MRSA detection and infection control, we recommend the following resources:
- Centers for Disease Control and Prevention (CDC): "Methicillin-resistant Staphylococcus aureus (MRSA) - General Information." An excellent resource for both professionals and the public. Visit CDC MRSA Page.
- World Health Organization (WHO): "Antimicrobial resistance." Provides a global perspective on the threat of resistance and the importance of diagnostics. Visit WHO AMR Page.
- Journal of Clinical Microbiology: Lamy, B., et al. (2020). "Performance of a new rapid culture-free molecular assay for the diagnosis of bloodstream infections." This type of research highlights the clinical impact of rapid molecular diagnostics for bacteremia. Read on JCM.
- Clinical and Laboratory Standards Institute (CLSI): CLSI provides globally recognized standards for laboratory testing, including guidelines for antimicrobial susceptibility testing and molecular diagnostics which inform the development of our kits. Visit CLSI Website.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







