Oct . 27, 2025 15:40 Back to list
CMV DNA Quantitative PCR Assay – Fast, Sensitive Viral Load
Inside the race for better CMV monitoring: Cmv Dna Quantitative Pcr you can actually run in the real world
When I visited a mid-sized transplant center earlier this year, one lab manager told me, “If CMV gets ahead of us, we lose precious days.” That’s the story across the industry: faster, traceable, and standardized Cmv Dna Quantitative Pcr has turned from “nice to have” into “critical.” Cowingene’s Cytomegalovirus Detection Kit (NATBox), made in NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China, has been popping up in procurement shortlists—so I dug in.
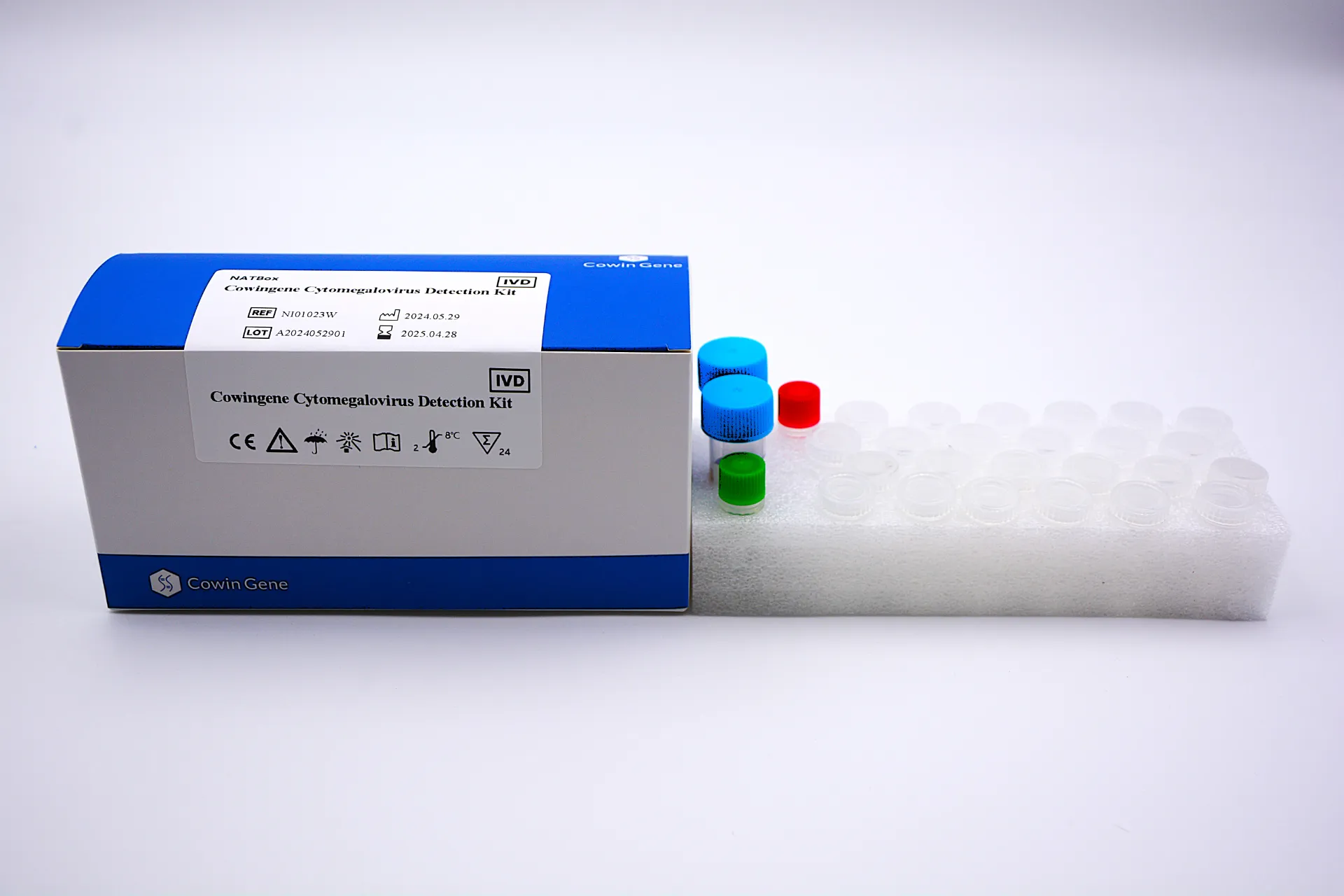
Industry trends and what matters now
Three threads dominate: adoption of WHO-standard IU/mL reporting, end-to-end traceability for audits (ISO 15189 vibes), and sample-flexible workflows. Actually, many customers say they want pragmatic wins: fewer tube swaps, faster turnaround, and “no drama” QC. The NATBox model leans into that with one-tube quantification and validated specimens across plasma, serum, urine, and whole blood.
Technical overview and process flow
Materials: pre-formulated master mix, primers/probes targeting CMV DNA, internal control, and calibration materials aligned (or alignable) to international standards. Methods: real-time PCR with quantitative curve fitting (typically 5–6 points), internal inhibition control, and software that reports in IU/mL. Testing standards: labs usually reference WHO International Standard for CMV DNA and CLSI guidelines for viral load quantification. Service life: typical shelf life ≈ 12–18 months at recommended storage (real-world use may vary). Industries: transplant centers, hematology/oncology, NICU follow-up, public-health surveillance.

Product specifications (field notes)
| Product name | Cowingene Cytomegalovirus Detection Kit (NATBox) |
| Analyte | CMV DNA, 1-tube quantitative format |
| Validated specimens | Plasma, Serum, Urine, Whole blood |
| Reporting unit | IU/mL (labs typically align to WHO IS; check your calibration) |
| Dynamic range | ≈ 2.0–7.0 log10 IU/mL (class-typical; site validation required) |
| LoD (indicative) | Around 100–300 IU/mL in class; confirm with in-house verification |
| Storage / stability | -20°C, avoid freeze-thaw; shelf life ≈ 12–18 months |
Application scenarios
- Pre-emptive monitoring in SOT/HSCT programs
- Post-therapy viral load tracking and resistance workups (paired with genotyping)
- NICU and congenital CMV follow-up (with careful matrix selection)
- Epidemiology studies where flexible matrices help
Vendor landscape (quick comparison)
| Vendor / Kit | Specimens | Automation | Typical LoD | Notes |
|---|---|---|---|---|
| Cowingene NATBox | Plasma/Serum/Urine/Whole blood | Open RT-PCR platforms | ≈100–300 IU/mL (site-dependent) | One-tube workflow; flexible matrices |
| Roche cobas CMV | Plasma | Fully integrated cobas | ≈70–150 IU/mL (public sources) | End-to-end traceability on cobas |
| Abbott RealTime CMV | Plasma | m2000/Alinity m | ≈100–200 IU/mL (public sources) | High-throughput hospital labs |
Customization, QC, and feedback
Labs often request calibration packs aligned to WHO IS and matrix-specific validation support. QC usually involves positive/negative controls plus an internal inhibition control. Users I spoke with liked the one-tube convenience and “fewer pipetting steps.” A few flagged that whole-blood precision can drift without rigorous extraction QC—fair point, and typical for Cmv Dna Quantitative Pcr across vendors.
Mini case snapshot
A 700-bed center switched to NATBox for urine and plasma to harmonize congenital and transplant workflows. After verification, their average TAT dropped by ~15%. Their infectious-disease lead told me, “It was the matrix flexibility that sold it,” though they kept a high-throughput platform for peak runs—sensible hybrid strategy.
Testing and compliance
- Traceable quantification to WHO International Standard for CMV DNA (lab-calibrated)
- Verification against CLSI viral-load guidelines; precision studies across ≥3 days recommended
- Operate within ISO 15189-accredited QMS where applicable
Bottom line: if you need a practical, sample-flexible Cmv Dna Quantitative Pcr kit and don’t want to be locked into a single closed system, NATBox is worth a validation run.
References
- WHO International Standard for Human Cytomegalovirus (NIBSC 09/162).
- CLSI MM06-A: Quantitation of Viral Load by Nucleic Acid Amplification Methods—Approved Guideline.
- ISO 15189: Medical laboratories—Requirements for quality and competence.
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







