Oct . 07, 2025 12:10 Back to list
Plasmodium Detection with Rapid PCR Accuracy—Why Us?
Cowingene NATBox: a field-tested view on plasmodium detection
When malaria flares, minutes matter. I’ve watched small clinics in border towns go from uncertainty to confident calls after a single run on a compact NAT instrument. That’s the promise of the Cowingene Plasmodium Detection Kit (NATBox): a tidy workflow for fast, lab-grade results—in places where shipping samples to a central lab just isn’t an option.
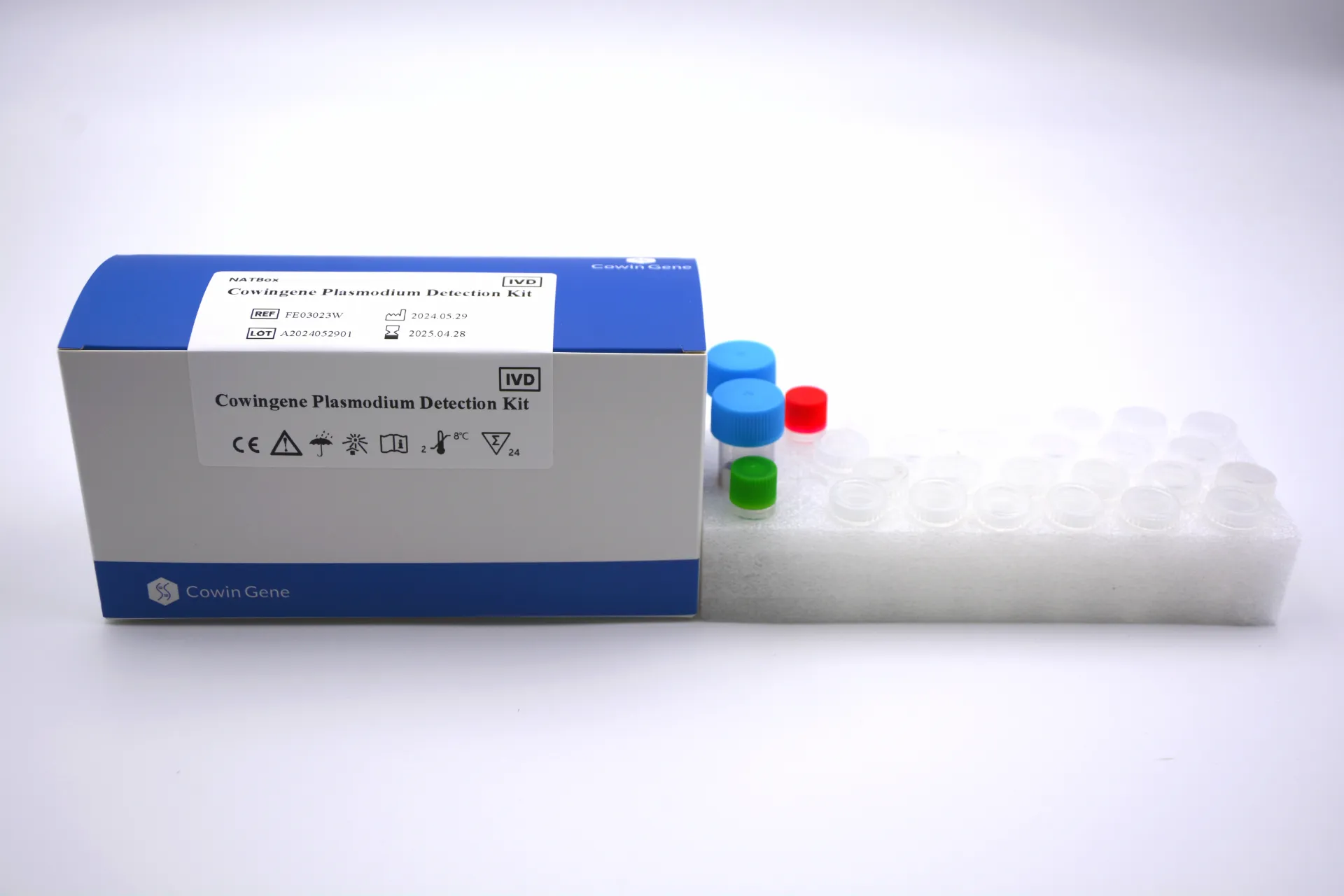
What’s inside and how it runs
Originating from NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China, the NATBox kit targets pan-Plasmodium genetic markers (typical assays focus on conserved 18S rRNA regions; some use mitochondrial sequences—real-world use may vary). Validated specimen: whole blood collected in EDTA or ACD tubes. One tube, one analyte: Plasmodium genus.
Typical process flow (kept practical):
- Materials: EDTA/ACD whole blood; provided reaction mix; nuclease-free consumables; NATBox instrument or compatible real-time PCR cycler.
- Methods: nucleic acid release/extraction (rapid prep or integrated), amplification (real-time PCR), fluorescence detection, automated call.
- Testing standards touchstones: CLSI EP17 for LoD studies, CLSI MM03 for molecular assay validation, lab accreditation under ISO 15189, QMS under ISO 13485. To be honest, these aren’t just badges—they shape how reliable your positives/negatives really are.
- Service life: kits are typically stored cold; shelf life commonly ≈ 12 months when stored as instructed (check the IFU; conditions matter).
- Industries: public health programs, travel clinics, emergency response, clinical labs, and yes—even donor screening support in some regions (policy-dependent).

Why teams pick it (and where it shines)
Speed is the headline. From sample to answer in under an hour is common—handy for triage when fevers spike. Multiplex-style pan-genus coverage reduces the guesswork. In outbreak logistics, less pipetting is more safety. Many customers say the “one tube, one target” simplicity keeps training time short. And, surprisingly, throughput scales: single-sample urgent runs at a roadside clinic, or batch runs back at district labs.
Product specs (practical view)
| Product | Cowingene Plasmodium Detection Kit (NATBox) |
| Target | Pan-Plasmodium DNA/RNA markers (genus level) |
| Specimen | Whole blood (EDTA or ACD) |
| Run time | ≈ 45–60 min (workflow-dependent) |
| LoD | Around low-copy detection per CLSI EP17-style studies (site results vary) |
| Storage / Shelf life | Cold-chain; typical shelf life ≈ 12 months (see IFU) |
| Certifications | Manufacturing QMS aligned with ISO 13485; labs often operate under ISO 15189 |
Vendor landscape (quick comparison)
| Vendor/Kit | Method | Time | Typical Use | Notes |
|---|---|---|---|---|
| Cowingene NATBox | Real-time PCR (pan-genus) | ≈ 45–60 min | Decentralized clinics, mobile teams | Compact workflow; low training burden |
| Generic qPCR kits | Multiplex qPCR | 60–90 min | Central labs, higher throughput | Flexible but needs skilled staff |
| RDTs (antigen) | Immunochromatography | 15–20 min | Screening, low-resource | Very fast; lower sensitivity vs NAAT |
Real-world scenarios and feedback
Border screening units told me they liked the “one-cart” footprint; power flickers aren’t deal-breakers when runs are short. A district lab in a coastal hotspot reported fewer indeterminate results after tightening pre-analytical steps—simple stuff like mixing EDTA blood properly. Actually, that’s consistent with best practices: good sampling beats fancy chemistry more often than we admit.
Case snapshot: a mobile team in a mining region used NATBox to triage febrile workers. Turnaround cut to under an hour, allowing immediate treatment pathways while microscopy confirmation arrived later. In fact, pairing plasmodium detection by NAAT with microscopy remains a smart strategy—fast rule-in, species detail later if needed.
Customization and support
- Customization: panel tweaks, kit size, and packaging for program rollouts—ask for regional regulatory alignment.
- Training: quick-start modules; SOP templates aligned to ISO 15189.
- Documentation: IFU with QC recommendations (positive/negative controls), and guidance for EP17-style LoD checks.
Bottom line: for teams needing dependable, fast plasmodium detection without a big-lab footprint, NATBox fits snugly into real workflows. Not perfect—nothing is—but it’s practical and, in the field, practical wins.
Authoritative references
- WHO. Malaria guidelines and policies. https://www.who.int/teams/global-malaria-programme/guidelines-and-policies/malaria
- CLSI EP17. Evaluation of Detection Capability. https://clsi.org/standards/products/method-evaluation/documents/ep17/
- ISO 13485:2016 Medical devices—Quality management systems. https://www.iso.org/standard/59752.html
- CDC. Malaria (DPDx). https://www.cdc.gov/dpdx/malaria/index.html
- ISO 15189:2022 Medical laboratories—Requirements for quality and competence. https://www.iso.org/standard/76677.html
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







