Nov . 02, 2025 23:30 Back to list
RSV Detection Kit – Rapid, Accurate, Easy-to-Use Testing
Rsv Detection Kit is a key solution in the healthcare industry, specifically within medical device and In Vitro Diagnostic Reagents. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
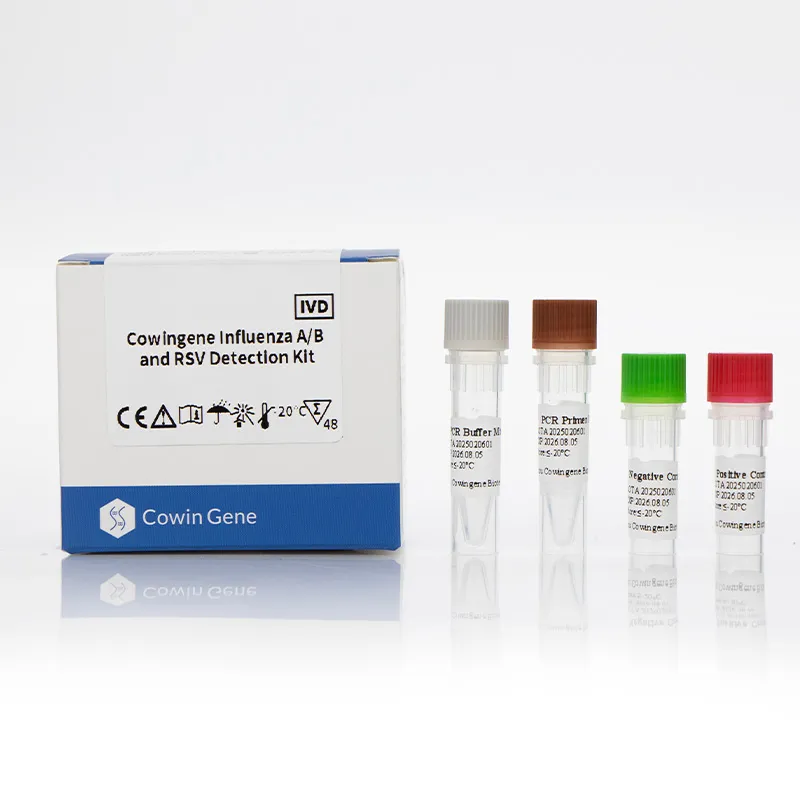
Table of Contents
- Rsv Detection Kit Overview
- Benefits & Use Cases of Rsv Detection Kit in In Vitro Diagnostic Reagents
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in healthcare
- Conclusion on Rsv Detection Kit from Taizhou Cowingene Biotech Co.,Ltd.
Rsv Detection Kit Overview
In the respiratory diagnostics landscape, an Rsv Detection Kit is an in vitro diagnostic reagent system developed to qualitatively detect respiratory syncytial virus (RSV) in upper respiratory specimens such as nasopharyngeal swabs or aspirates. For laboratories and healthcare networks that face overlapping symptoms with influenza and other respiratory pathogens, a reliable rsv kit shortens the diagnostic journey and supports confident clinical decisions. Taizhou Cowingene Biotech Co.,Ltd. engineers the kit to integrate smoothly with common nucleic acid extraction workflows and real-time PCR platforms, enabling laboratories to scale RSV detection without reconfiguring their infrastructure.
- Product relevance: purpose-built for RSV detection in clinical specimens, supporting patient triage during peak respiratory seasons and vulnerable-population monitoring.
- Technical background: optimized primers/probes and internal control help reduce false negatives due to inhibition; typical workflow fits within standard RT‑qPCR cycles for efficient turnaround times.
- Reliable manufacturer: Taizhou Cowingene Biotech Co.,Ltd. delivers consistent lot-to-lot performance from a quality-managed manufacturing environment, backed by responsive technical support for B2B partners.
Benefits & Use Cases of Rsv Detection Kit in In Vitro Diagnostic Reagents
For clinical labs, reference centers, and integrated delivery networks, a high-performance Rsv Detection Kit streamlines testing pathways from sample check‑in to report release. It supports syndromic testing algorithms, including differential diagnosis among RSV, influenza A/B, and other respiratory pathogens where multiplex workflows are used. Distributors also benefit from a stable portfolio anchor that addresses seasonal demand spikes with dependable supply and technical documentation. Competitive advantages include strong analytical sensitivity/specificity, compatibility with widely used qPCR instruments, and built‑in controls that simplify validation.
- Applications: hospital labs, urgent care networks, public health surveillance, and occupational health programs requiring scalable RSV detection.
- Features: streamlined protocol, clear interpretation criteria, and throughput options for batch or on‑demand testing, helping reduce repeat runs and hands‑on time.
- Expertise: Taizhou Cowingene Biotech Co.,Ltd. understands B2B priorities—robust QC, documentation, and training resources—so teams can deploy the rsv kit quickly and maintain consistent performance across sites.
Cost, Maintenance & User Experience
Total cost of ownership for an Rsv Detection Kit extends beyond the per‑test price to include training, instrument compatibility, workflow efficiency, and waste. By offering an intuitive protocol and compatibility with common extraction and RT‑qPCR systems, Taizhou Cowingene Biotech Co.,Ltd. helps labs avoid additional capital expenditures and reduces onboarding time. Fewer repeat tests, clear result interpretation, and reliable internal controls contribute to lower operational costs and a stronger ROI—especially during high‑volume respiratory seasons.
- Durability and shelf life support inventory planning, with packaging designed to minimize reagent loss and preserve performance across the product’s lifetime.
- Customer feedback from medical device sector partners highlights reduced hands‑on time, consistent lot performance, and dependable technical support—key factors for multi‑site standardization.
Sustainability & Market Trends in healthcare
RSV remains a priority for healthcare systems due to seasonal surges, the aging population, and pediatric vulnerability. Market trends point to continued growth in molecular diagnostics, emphasis on multiplex respiratory panels, and greater attention to supply resilience. Within this context, procurement teams seek partners who balance performance with responsible operations. Taizhou Cowingene Biotech Co.,Ltd. advances eco‑conscious practices by focusing on efficient packaging, streamlined cold‑chain logistics, and quality-by-design processes that reduce waste from rework or invalid runs.
- Regulatory and quality expectations are rising, placing value on robust documentation, traceability, and post‑market support—areas where Cowingene invests to support long‑term partnerships.
- Forward‑thinking stance: the company aligns product development with lab automation, data integrity, and sustainability goals, helping stakeholders meet both clinical and ESG objectives.
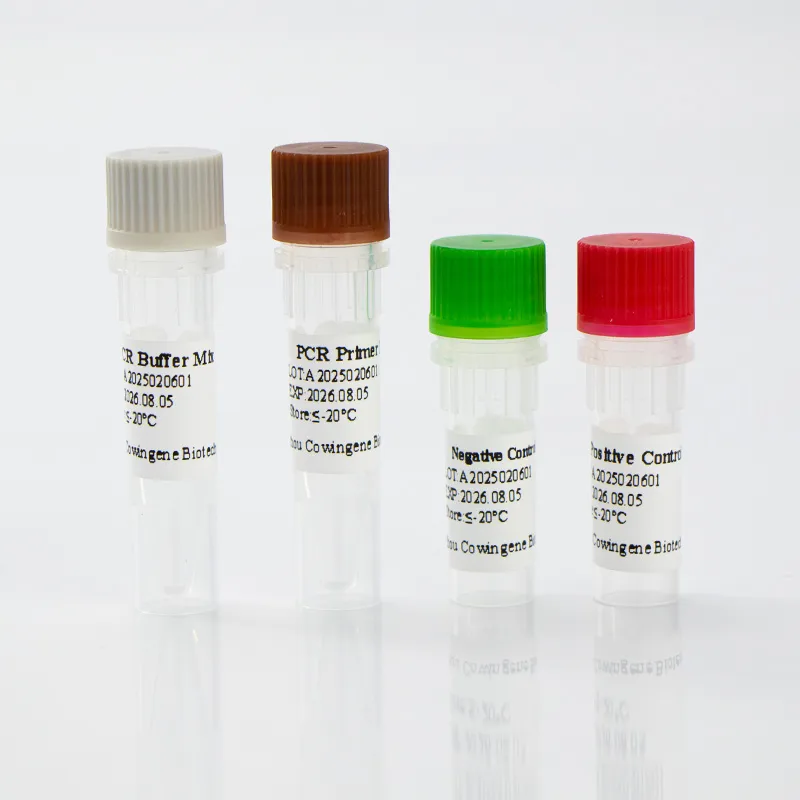
Conclusion on Rsv Detection Kit from Taizhou Cowingene Biotech Co.,Ltd.
For B2B decision makers, the Rsv Detection Kit offers reliable RSV detection, efficient workflows, and strong operational value within the In Vitro Diagnostic Reagents segment. Taizhou Cowingene Biotech Co.,Ltd. stands out for consistent quality, practical documentation, and responsive technical support—delivering a trusted rsv kit that scales with your testing demand. Contact us: email: info@cowingene.com — Visit our website: https://www.cowingene.com
Related PRODUCTS
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
NewsNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
NewsNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
NewsNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
NewsNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
NewsNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
NewsNov.04,2025







