Sep . 23, 2025 14:25 Back to list
RSV Detection Kit: Fast, Accurate Results for Respiratory Health
Advancements in RSV Detection: A Comprehensive Overview for B2B Stakeholders
Respiratory Syncytial Virus (RSV) continues to pose a significant public health challenge, particularly for vulnerable populations such as infants and the elderly. Accurate and rapid diagnosis is critical for effective patient management, infection control, and epidemiological surveillance. The market for advanced diagnostic solutions, particularly in vitro diagnostic (IVD) assays, is experiencing robust growth driven by increasing demand for point-of-care testing (POCT) and multiplex platforms capable of differentiating respiratory pathogens.
Industry trends indicate a strong shift towards highly sensitive, specific, and user-friendly diagnostic tools. The integration of molecular techniques, such as real-time RT-PCR, with lyophilized reagent formats is revolutionizing diagnostic workflows by enhancing stability, simplifying logistics, and reducing cold chain requirements. This comprehensive overview explores the technical intricacies, application benefits, and strategic considerations surrounding modern rsv detection kit solutions, specifically focusing on lyophilized multiplex platforms.
Process Flow: Manufacturing a High-Quality Lyophilized RSV Detection Kit
The production of a sophisticated molecular diagnostic kit, such as the Cowingene Influenza A/B and rsv detection kit (Lyophilized), involves a meticulously controlled manufacturing process designed to ensure reagent stability, assay performance, and user convenience. This process adheres strictly to international quality management systems, including ISO 13485:2016 for medical devices and relevant aspects of current Good Manufacturing Practices (cGMP), to guarantee consistent product quality and regulatory compliance.
- Reagent Formulation and Preparation: This initial stage involves the precise formulation of all assay components, including primers, probes, reverse transcriptase, Taq polymerase, dNTPs, and proprietary stabilizers. Raw materials are sourced from qualified suppliers and undergo stringent incoming quality control (IQC) to verify purity and absence of contaminants. The genetic sequences for primers and probes are rationally designed to target conserved regions of RSV, Influenza A, and Influenza B viral genomes, ensuring high specificity and sensitivity.
- Dispensing and Filling: The formulated liquid reagent mixture is aseptically dispensed into individual reaction tubes or wells of multi-well plates. This step is performed in cleanroom environments (e.g., ISO Class 7) to prevent microbial contamination. Automated precision liquid handling systems ensure accurate and consistent fill volumes, critical for assay reproducibility.
- Lyophilization (Freeze-Drying): This is a critical process where the dispensed liquid reagents are subjected to freeze-drying.
- Freezing: The reagents are rapidly frozen to extremely low temperatures (typically -40°C to -80°C) to solidify the water, forming a stable ice matrix.
- Primary Drying (Sublimation): Under vacuum, the temperature is carefully raised, causing the ice to sublimate directly into water vapor, removing approximately 95% of the water.
- Secondary Drying (Desorption): The remaining unfrozen water molecules are removed through desorption, achieving extremely low moisture content (typically
- Sealing and Packaging: After lyophilization, the tubes/plates are hermetically sealed, often under inert gas, to protect the dried reagents from moisture and oxygen. They are then packaged with desiccant and appropriate labeling, detailing batch information, expiry dates, and usage instructions.
- Quality Control and Release Testing: Every manufacturing batch undergoes rigorous QC testing. This includes:
- Analytical Testing: pH, conductivity, moisture content, visual inspection.
- Functional Performance Testing: Evaluation of sensitivity (Limit of Detection - LoD), specificity, accuracy, precision, and reproducibility using positive and negative controls, and clinical samples. This ensures the rsv kit performs to specification.
- Stability Testing: Accelerated and real-time stability studies determine the shelf life of the lyophilized product, typically guaranteeing a service life of 12-24 months at ambient temperatures.
The target industries for these kits are broad, encompassing clinical diagnostic laboratories, public health agencies, hospital infectious disease departments, and research institutions. The inherent advantages of lyophilized formats, such as energy saving through reduced cold chain reliance and enhanced corrosion resistance (elimination of liquid degradation pathways), make them ideal for deployment in diverse geographical and resource-constrained settings.
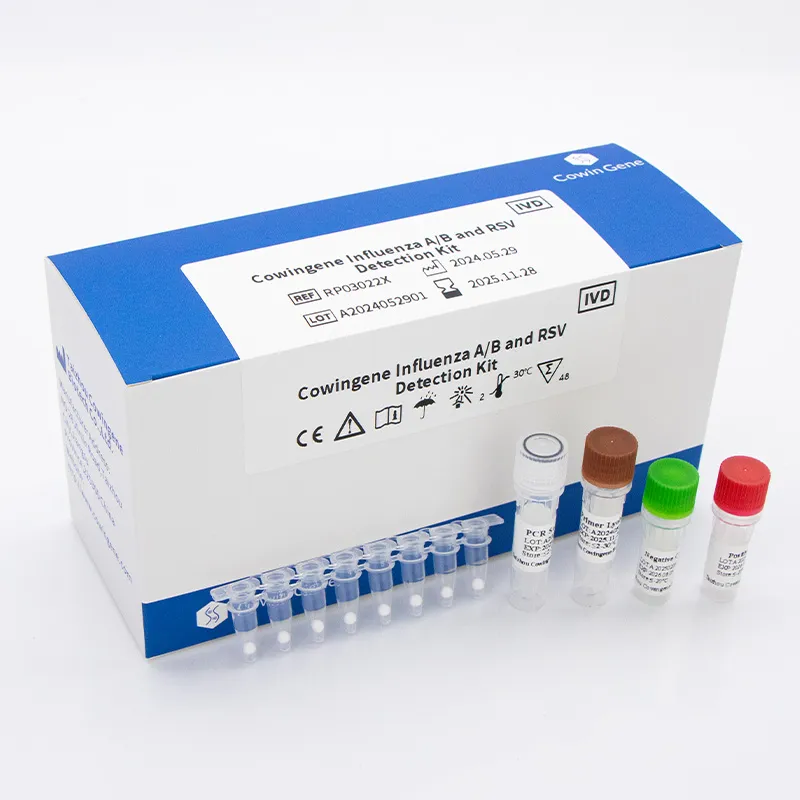
Figure 1: Automated dispensing and lyophilization chamber for diagnostic reagents.
Technical Specifications and Parameters of Lyophilized RSV Detection Kits
Modern rsv detection kits, especially those incorporating lyophilized reagents, are designed to deliver high performance in demanding clinical environments. Key technical parameters define their efficacy and reliability.
| Parameter | Specification (Cowingene Influenza A/B and RSV Detection Kit Example) |
|---|---|
| Assay Type | Real-time RT-PCR (Multiplex for Flu A, Flu B, RSV) |
| Sample Type | Nasopharyngeal swabs, Oropharyngeal swabs, Bronchoalveolar lavage (BAL), Sputum |
| Limit of Detection (LoD) | RSV: |
| Specificity | >99% (No cross-reactivity with common respiratory pathogens and commensals) |
| Clinical Sensitivity | >95% (Compared to reference molecular methods) |
| Clinical Specificity | >98% (Compared to reference molecular methods) |
| Reaction Volume | Typically 20-25 µL |
| Reaction Time | Approx. 60-90 minutes (Post RNA extraction) |
| Storage Conditions | 2-30°C (Ambient temperature); avoid direct sunlight |
| Shelf Life | 12-24 months from manufacturing date |
| Compatible Instruments | Various qPCR systems (e.g., Applied Biosystems 7500 series, Bio-Rad CFX96, Roche LightCycler 480) |
These specifications underscore the advanced engineering behind a reliable rsv detection kit. The lyophilized format significantly contributes to extending the shelf life and reducing storage complexities, a critical factor for global distribution and deployment in diverse healthcare settings.
Application Scenarios and Technical Advantages
The versatility and robustness of lyophilized rsv detection kit solutions enable their deployment across a wide array of application scenarios, each benefiting from their distinct technical advantages.
Typical Application Scenarios:
- Clinical Diagnostics: Rapid and accurate differentiation of RSV, Influenza A, and Influenza B in patients presenting with acute respiratory infections. This aids clinicians in making timely treatment decisions, especially concerning antiviral therapies and infection control measures.
- Epidemiological Surveillance: Public health laboratories utilize these kits for monitoring seasonal respiratory virus activity, identifying outbreaks, and tracking the prevalence of specific strains. This data is vital for public health interventions and vaccine strategies.
- Emergency Departments & Urgent Care: The ability to perform rapid multiplex testing allows for quicker patient triage, reducing the spread of highly contagious viruses and optimizing resource allocation.
- Resource-Limited Settings: The ambient storage requirement and simplified workflow make lyophilized kits ideal for remote clinics or areas with unreliable cold chain infrastructure, facilitating broader access to advanced molecular diagnostics.
Technical Advantages:
- Enhanced Reagent Stability: Lyophilization removes water, a primary factor in reagent degradation. This grants significantly longer shelf life (12-24 months) even at room temperature (2-30°C), reducing the need for costly cold storage and transportation. This is a major advantage over traditional liquid reagents which often require -20°C storage.
- Simplified Workflow and Reduced Error Rate: Pre-aliquoted, dried reagents minimize pipetting steps, reducing hands-on time and the potential for human error or cross-contamination. Users simply rehydrate the pellet with extracted nucleic acid and master mix.
- Cost-Effectiveness and Logistics: Reduced reliance on cold chain logistics translates into significant cost savings on shipping and storage. The compact format also optimizes storage space.
- Multiplexing Capabilities: Simultaneous detection of multiple pathogens (e.g., RSV, Flu A, Flu B) from a single sample provides a comprehensive diagnostic picture in a single reaction, saving time and sample material.
- High Sensitivity and Specificity: Designed with optimized primer/probe sets and advanced enzyme technologies, these kits deliver superior analytical performance, detecting even low viral loads with high precision.
Comparison: Lyophilized vs. Traditional Liquid RSV Detection Kits
| Feature | Lyophilized Kit (e.g., Cowingene) | Traditional Liquid Kit |
|---|---|---|
| Storage Temperature | 2-30°C (Ambient) | -20°C or colder (Frozen) |
| Shelf Life | 12-24 months | 6-12 months |
| Cold Chain Requirement | Minimal/None | High |
| Shipping Cost | Lower | Higher (requires cold shipping) |
| Workflow Complexity | Simplified (fewer pipetting steps) | More complex (multiple pipetting steps) |
| Risk of Contamination/Error | Lower | Higher |
These advantages collectively position lyophilized rsv kit solutions as a cornerstone for modern molecular diagnostics, improving accessibility and efficiency in respiratory pathogen detection.
Vendor Comparison and Customized Solutions
The market for respiratory diagnostic kits is competitive, with numerous vendors offering various solutions. When evaluating a rsv detection kit, B2B buyers consider not only the technical specifications but also factors like regulatory clearances, support, and the potential for customization.
Key Factors in Vendor Comparison:
- Regulatory Status: Compliance with international standards (CE-IVD, FDA EUA/clearance) is paramount, ensuring product safety and performance.
- Performance Data: Robust clinical validation studies, clear LoD, sensitivity, and specificity data are crucial for evidence-based decision making.
- Ease of Use and Workflow Integration: Kits that minimize hands-on time and integrate seamlessly with existing lab equipment (e.g., RNA extraction platforms, qPCR thermocyclers) offer significant operational benefits.
- Supply Chain Reliability: The ability of a vendor to consistently supply kits, especially during peak seasons or epidemics, is critical. Lyophilized formats inherently contribute to supply chain resilience due to less stringent storage.
- Technical Support and Training: Comprehensive post-sales support, including technical assistance, troubleshooting, and training, adds significant value.
Customized Solutions:
Beyond standard offerings, reputable manufacturers like Cowingene often provide customized solutions tailored to specific client needs. This can include:
- Panel Expansion: Development of multiplex panels to include additional respiratory pathogens (e.g., hMPV, Adenovirus, Parainfluenza viruses) alongside Flu A/B and RSV.
- Format Adaption: Customization for different PCR instrument platforms or integration into automated liquid handling systems.
- Bulk Packaging: Offering reagents in bulk formats for high-throughput laboratories or for OEM partnerships.
- Private Labeling: Manufacturing kits under a client's own brand, while maintaining the underlying quality and performance standards of the core rsv detection technology.
Such flexibility ensures that specific operational and regulatory requirements of diverse clients can be met, fostering strong, long-term B2B partnerships.
Real-World Application Case Studies
The efficacy and practical benefits of advanced rsv detection kits are best illustrated through real-world applications and customer experiences. Cowingene has a track record of successful deployments globally, demonstrating the reliability and impact of its diagnostic solutions.
Case Study 1: Large Hospital Network in Southeast Asia
A major hospital network, comprising 15 facilities across a rapidly developing Southeast Asian nation, faced significant challenges with respiratory pathogen diagnosis. Traditional liquid PCR kits required extensive cold chain infrastructure, leading to high logistics costs and occasional reagent degradation in remote clinics. They sought a robust, stable, and multiplex solution.
- Solution Implemented: Cowingene Influenza A/B and RSV Detection Kit (Lyophilized).
- Outcome: Within six months of implementation, the network reported a 30% reduction in cold chain logistics costs and a significant decrease in reagent wastage. The ambient stability of the lyophilized kit allowed for decentralized testing in smaller clinics, dramatically reducing turnaround times for RSV and Flu A/B results from 48 hours to less than 6 hours. This improved patient flow and enabled earlier infection control measures. Clinical feedback highlighted the ease of use and consistent performance, even for less experienced laboratory personnel.
Case Study 2: Public Health Surveillance Program in Europe
A national public health agency in Europe required a reliable and standardized molecular assay for seasonal respiratory virus surveillance. Their existing workflow was fragmented, utilizing different single-target assays from various manufacturers, leading to inconsistencies and increased labor burden. They aimed to consolidate and streamline their testing for Flu A/B and RSV.
- Solution Implemented: Custom-tailored Cowingene multiplex rsv kit, adapted for their specific high-throughput qPCR platforms and integrated with their LIS (Laboratory Information System).
- Outcome: The agency achieved a 40% improvement in throughput efficiency due to the multiplexing capability and streamlined workflow. Data consistency across their national laboratory network improved, leading to more robust epidemiological reporting. The lyophilized format ensured long-term reagent availability and simplified emergency stockpiling for future outbreaks. The technical support provided by Cowingene during the integration phase was cited as instrumental to the successful transition.
Figure 2: Real-time PCR setup utilizing a multiplex rsv detection kit in a clinical laboratory.
Ensuring Trustworthiness: FAQ, Lead Time, Warranty, and Support
Frequently Asked Questions (FAQ)
Q: What is the primary advantage of a lyophilized rsv detection kit over a liquid one?
A: Lyophilized kits offer superior long-term stability at ambient temperatures (2-30°C), eliminating the need for cold chain storage and transport, which reduces costs and logistical complexities. They also simplify workflow by minimizing pipetting steps.
Q: Is RNA extraction necessary before using this kit?
A: Yes, nucleic acid extraction (RNA extraction) from the patient sample is a prerequisite step to remove inhibitors and concentrate viral RNA, ensuring optimal PCR performance. The kit works downstream of standard RNA extraction protocols.
Q: What qPCR instruments are compatible with the Cowingene RSV/Flu kit?
A: Our kits are validated for use on a wide range of popular real-time PCR systems, including but not limited to Applied Biosystems 7500 series, Bio-Rad CFX96, and Roche LightCycler 480. Please contact our technical support for specific instrument compatibility inquiries.
Q: How does Cowingene ensure the quality and reliability of its rsv detection kits?
A: We adhere to stringent quality management systems (ISO 13485:2016). All raw materials are rigorously screened, and every batch undergoes comprehensive quality control testing for sensitivity, specificity, accuracy, and stability before release. Our manufacturing processes are designed to minimize contamination and ensure product consistency.
Lead Time and Fulfillment
Standard lead time for our Cowingene Influenza A/B and RSV Detection Kits typically ranges from 2-4 weeks, depending on order volume and current stock levels. For large-scale orders or customized solutions, a dedicated account manager will provide a precise fulfillment schedule. We maintain robust inventory levels to support urgent demands and seasonal surges, leveraging our efficient logistics network for timely global delivery.
Warranty Commitments
Cowingene warrants that its rsv detection products will meet the performance specifications outlined in the product inserts when stored and used according to instructions, until the stated expiration date. Any product found to be non-conforming within this period will be replaced or refunded. Our commitment to quality is unwavering, backed by a comprehensive warranty policy.
Customer Support and Technical Assistance
Our dedicated customer support team and technical specialists are available to assist with product inquiries, assay optimization, troubleshooting, and training. We offer multi-channel support including phone, email, and online resources. With years of experience in molecular diagnostics, our experts ensure that clients receive prompt and effective assistance to maximize the utility of their Cowingene products.
Conclusion
The evolution of molecular diagnostics, particularly in the realm of respiratory virus detection, highlights the critical role of innovative solutions like the lyophilized rsv detection kit. By combining high analytical performance with unparalleled stability and ease of use, these kits address key challenges in clinical diagnostics and public health surveillance. For B2B partners, investing in such advanced platforms ensures reliable, efficient, and cost-effective detection capabilities, ultimately contributing to better patient outcomes and more effective disease management strategies globally.
References
- Centers for Disease Control and Prevention. (2023). Respiratory Syncytial Virus (RSV) Information. Retrieved from https://www.cdc.gov/rsv/index.html
- World Health Organization. (2022). WHO guidance for surveillance and laboratory testing for respiratory syncytial virus (RSV) infection. Retrieved from https://www.who.int/publications/i/item/WHO-2022-RSV-surveillance-testing-guidance-0.1
- ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes. International Organization for Standardization.
- Journal of Clinical Microbiology. (Various Issues). Articles on molecular diagnostics and respiratory virus detection.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







