Nov . 03, 2025 00:00 Back to list
Chlamydia DNA Detection Meaning | Accurate, Fast PCR
Chlamydia Dna Detection Meaning is a key solution in the medical device industry, specifically within In vitro diagnostic products and Molecular Diagnostics. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
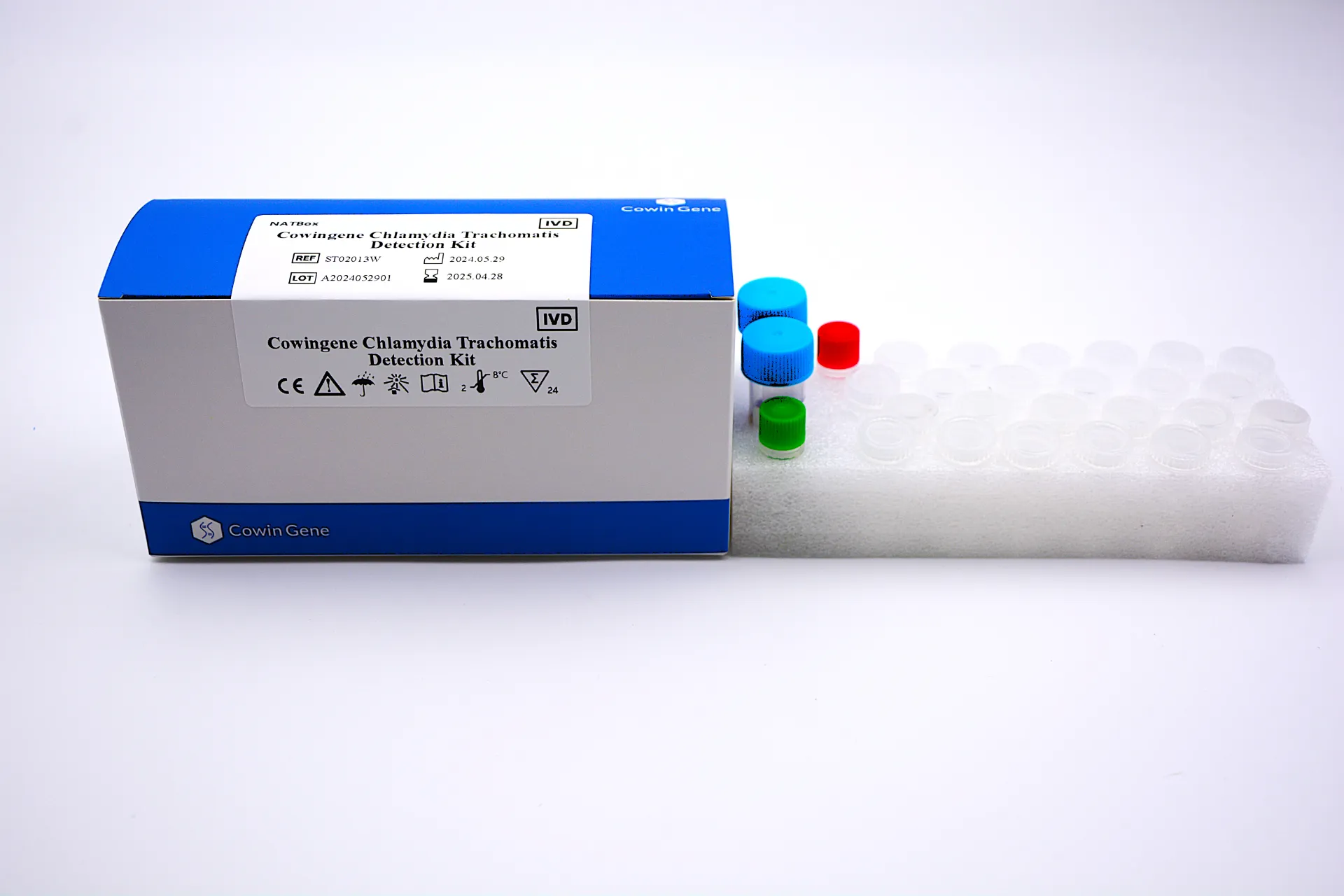

Table of Contents
- Chlamydia Dna Detection Meaning Overview
- Benefits & Use Cases of Chlamydia Dna Detection Meaning in Molecular Diagnostics
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in medical device
- Conclusion on Chlamydia Dna Detection Meaning from Taizhou Cowingene Biotech Co.,Ltd.
Chlamydia Dna Detection Meaning Overview
For B2B professionals, Chlamydia Dna Detection Meaning encompasses the scientific and operational rationale for using nucleic acid amplification testing (NAAT) to find Chlamydia trachomatis in clinical specimens. In practice, a chlamydia dna pcr test amplifies target gene regions of C. trachomatis and detects them in real time, enabling high analytical sensitivity and specificity compared with culture or antigen assays. Taizhou Cowingene Biotech Co.,Ltd. delivers this capability through an expertly engineered kit designed for routine diagnostic workflows.
- Product definition: A real-time PCR solution for pcr for chlamydia trachomatis in urine, urogenital swabs, and other validated sample types—optimized for common qPCR platforms.
- Technical background: Conserved-target primer/probe design, included internal control, positive/negative controls, and contamination-resistant workflows improve confidence in results and interpretation.
- Manufacturer credibility: Taizhou Cowingene Biotech Co.,Ltd. is a reliable IVD supplier with robust QC systems, batch traceability, and application support aligned to molecular diagnostics best practices.
Benefits & Use Cases of Chlamydia Dna Detection Meaning in Molecular Diagnostics
In molecular labs, Chlamydia Dna Detection Meaning translates into actionable advantages: fast, sensitive NAAT results that support screening programs, contact tracing, and targeted therapy. The Cowingene kit enables laboratories to standardize NAAT across different throughput needs—supporting sexual health clinics, hospital core labs, and public health networks with scalable batching and streamlined interpretation.
- Applications: Routine STI screening, reflex testing after indeterminate antigen results, and surveillance projects requiring consistent, comparable qPCR data.
- Competitive advantages: Clear workflow instructions, internal process controls, strong analytical performance, and compatibility with widely used qPCR instruments reduce onboarding and validation time.
- Expertise: Taizhou Cowingene Biotech Co.,Ltd. provides technical documentation, training, and responsive support—helping labs implement chlamydia dna pcr test protocols efficiently and confidently.
Cost, Maintenance & User Experience
Total cost of ownership for NAAT programs extends beyond per-test pricing to include hands-on time, repeat rates, training, and instrument compatibility. The Cowingene chlamydia dna pcr test is designed to minimize repeat testing via robust internal controls and clear result interpretation, improving ROI. Reagent stability under recommended storage and intuitive kit layout streamline daily maintenance and inventory management for IVD labs.
- TCO and durability: Efficient workflows reduce labor burden; standardized components support predictable procurement and lower waste.
- User feedback: Customers in hospital and reference labs highlight straightforward setup, consistent controls, and smooth integration into existing PCR for Chlamydia trachomatis panels—translating to fewer invalids and faster turnaround times.
Sustainability & Market Trends in medical device
The STI diagnostics market continues to shift toward NAAT as the clinical gold standard, driven by public health initiatives, antimicrobial stewardship, and the need for rapid, reliable detection. Regulatory expectations emphasize quality systems, traceability, and result integrity. Within this context, Chlamydia Dna Detection Meaning aligns with evidence-based care pathways and digital laboratory ecosystems that prioritize data accuracy and operational efficiency.
- Sustainability: Cowingene focuses on efficient packaging, clear IFU design that reduces repeat testing, and robust lot consistency—supporting lower material waste and more sustainable testing operations.
- Forward-thinking stance: Taizhou Cowingene Biotech Co.,Ltd. continuously refines assay design and documentation to meet evolving guidelines in molecular diagnostics, positioning partners to scale responsibly and remain audit-ready.
Conclusion on Chlamydia Dna Detection Meaning from Taizhou Cowingene Biotech Co.,Ltd.
Chlamydia Dna Detection Meaning captures the value of NAAT-based workflows that deliver timely, precise results for Chlamydia trachomatis. For B2B decision makers across medical device, IVD, and Molecular Diagnostics, Taizhou Cowingene Biotech Co.,Ltd. provides a dependable, well-supported chlamydia dna pcr test that integrates seamlessly into modern labs. Partner with a manufacturer committed to quality, consistency, and service. Contact us: email: info@cowingene.com — Visit our website: https://www.cowingene.com
Related PRODUCTS
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
NewsNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
NewsNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
NewsNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
NewsNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
NewsNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
NewsNov.04,2025







