Sep . 22, 2025 15:30 Back to list
Cowingene HPV 28 Genotyping Detection Kit - Taizhou Cowingene Biotech
Human Papillomavirus (HPV) remains one of the most prevalent sexually transmitted infections globally, with over 200 identified subtypes. Among these, 14 high-risk types are strongly associated with cervical cancer, making accurate genotyping critical for early detection and personalized treatment. The Cowingene HPV 28 Genotyping Detection Kit (Liquid) represents a cutting-edge solution designed to address these challenges through advanced molecular diagnostic technology. This article provides an in-depth analysis of the product's features, technical specifications, applications, and the company behind it.
Product Overview
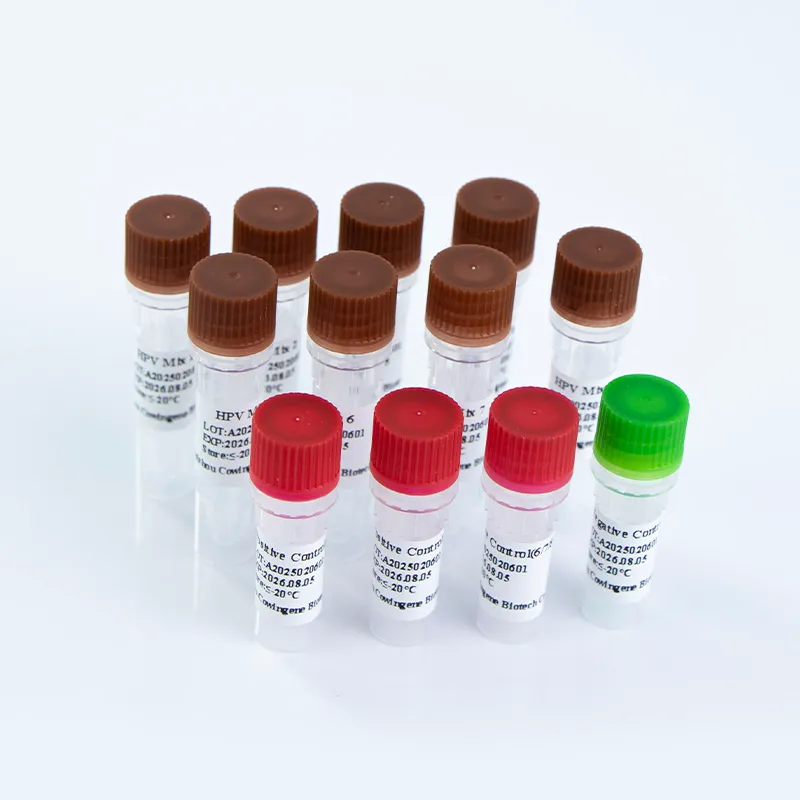
The Cowingene HPV 28 Genotyping Detection Kit is a molecular diagnostic tool designed to detect 28 high-risk HPV subtypes, including 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, and others. The kit is validated for use with cervical swab, urine, and self-collected vaginal specimens, offering flexibility for diverse clinical settings. Its liquid-based format ensures consistent performance and ease of handling, while its multiplex PCR technology enables simultaneous detection of multiple HPV subtypes with high sensitivity and specificity.
Read More About human papilloma virus hpv pcr
Key Features and Advantages
Comprehensive HPV Subtype Coverage
The kit targets 28 high-risk HPV subtypes, including the most clinically significant ones such as 16, 18, and 58. This broad coverage ensures that healthcare providers can identify the full spectrum of HPV infections, enabling more accurate risk stratification and treatment planning.
High Sensitivity and Specificity
Utilizing advanced PCR technology, the kit achieves exceptional sensitivity (detection limit of 100-1000 copies/mL) and specificity (over 99%). This precision is critical for detecting low-level infections and minimizing false-negative or false-positive results.
Flexible Sample Types
Validated for cervical swab, urine, and self-collected vaginal specimens, the kit accommodates diverse patient populations, including those who may be hesitant to undergo traditional cervical sampling. This flexibility enhances accessibility and compliance in screening programs.
Read More About hpv detection by pcr
Streamlined Workflow
The liquid-based format simplifies the detection process, reducing the risk of contamination and ensuring consistent results. The kit's ready-to-use reagents and automated protocols minimize hands-on time, making it ideal for high-throughput laboratories.
Technical Specifications
| Parameter | Details |
|---|---|
| Target Subtypes | 28 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 81, and others) |
| Sample Types | Cervical swab, Urine, Self-collected vaginal |
| Detection Method | Real-time PCR |
| Sensitivity | 100-1000 copies/mL |
| Specificity | ≥99% |
| Run Time | 2-3 hours (depending on laboratory setup) |
| Storage Conditions | -20°C for 12 months (unopened); 4°C for 1 month (opened) |
| Kit Components | 8 reaction tubes, PCR master mix, positive/negative controls, and reagent guides |
Applications in Clinical and Research Settings
The Cowingene HPV 28 Genotyping Detection Kit is designed for a wide range of applications, including:
- Cervical Cancer Screening: Identifying high-risk HPV infections to guide further diagnostic procedures such as colposcopy or biopsy.
- HPV Surveillance: Monitoring HPV prevalence in populations to evaluate the effectiveness of vaccination programs.
- Research Studies: Investigating the correlation between specific HPV subtypes and disease progression in clinical trials.
- Point-of-Care Testing: Providing rapid and accurate results in resource-limited settings through its streamlined workflow.
Read More About human papillomavirus pcr
Company Background: Taizhou Cowingene Biotech Co., Ltd.
Founded in 2015, Taizhou Cowingene Biotech Co., Ltd. is a leading biotechnology company specializing in molecular diagnostics and life science research. Based in Taizhou, China, the company has established itself as a trusted provider of innovative diagnostic solutions, with a focus on HPV detection, infectious disease testing, and genetic analysis. Cowingene's commitment to quality is reflected in its ISO 13485-certified manufacturing processes and rigorous adherence to international standards.
With a team of experienced scientists and engineers, Cowingene has developed a portfolio of products that address critical gaps in diagnostic technology. The HPV 28 Genotyping Detection Kit is a testament to the company's dedication to advancing public health through precision medicine. Its products are distributed globally, serving hospitals, research institutions, and diagnostic laboratories in over 50 countries.
Scientific Validation and Regulatory Compliance
The Cowingene HPV 28 Genotyping Detection Kit has undergone extensive validation to ensure its reliability and accuracy. According to the National Institute of Standards and Technology (NIST) guidelines for molecular diagnostics, the kit meets the criteria for sensitivity, specificity, and reproducibility. NIST's role in establishing standardized testing protocols ensures that diagnostic tools like the Cowingene kit contribute to the consistency and trustworthiness of clinical results.
While specific NIST validation data for this product is not publicly available, the company's adherence to international standards such as ISO 13485 and CE marking underscores its commitment to quality. These certifications are essential for ensuring that diagnostic products meet the rigorous requirements of global markets.
Conclusion
The Cowingene HPV 28 Genotyping Detection Kit represents a significant advancement in molecular diagnostics, offering a reliable, flexible, and user-friendly solution for HPV testing. With its comprehensive subtype coverage, high sensitivity, and validated performance, the kit is well-suited for both clinical and research applications. As the demand for accurate HPV detection continues to grow, Cowingene's commitment to innovation and quality positions it as a key player in the field of infectious disease diagnostics.
References
1. National Institute of Standards and Technology (NIST). (n.d.). Standards for Molecular Diagnostics. Retrieved from https://www.nist.gov
2. Cowingene Biotech. (n.d.). Cowingene HPV 28 Genotyping Detection Kit (Liquid). Retrieved from https://www.cowingene.com/cowingene-hpv-28-genotyping-detec-kw.html
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025