Oct . 17, 2025 11:55 Back to list
Detection Chlamydia trachomatis PCR Kit - Fast, Accurate
Practical notes from the lab bench: panel testing for fast, confident detection
In the past five years, sexual health labs have quietly shifted toward multiplex panels—less retesting, fewer callbacks, more answers in one go. A good example is detection chlamydia trachomatis bundled with 13 other analytes in a single workflow. To be honest, what wins day-to-day is not flashy chemistry, it’s predictable turnarounds and clean reports that clinicians trust.
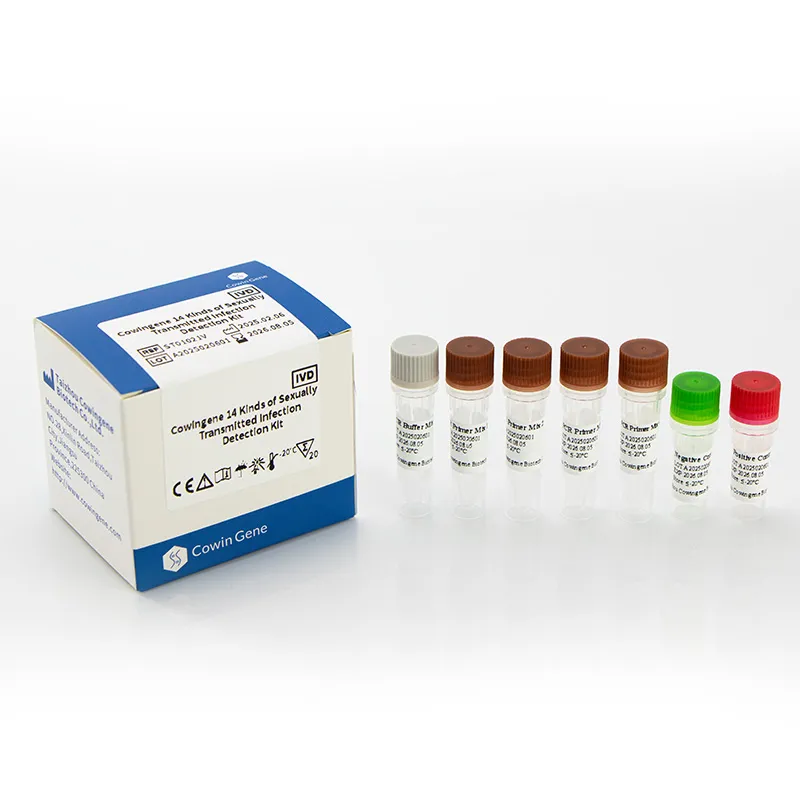
Product snapshot: Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid)
Origin: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China. REF: ST01021V. Validated specimen types include cervical and anorectal swabs, urine, and self-collected vaginal samples. Targets in 4 tubes: CT, NG, HSV1, HSV2, UU, UP, MH, MG, CA, GV, TV, GBS, HD, TP. It’s a practical roster for real clinics—nothing exotic, everything encountered weekly.

Why it matters now
Industry trend check: more NAAT-based panels; reflex rules built into LIS; and increasing demand for self-collected specimens. Clinicians want same-day calls on detection chlamydia trachomatis plus NG and MG, because treatment pathways diverge. Surprisingly, panels can lower total cost per answer by cutting repeat visits.
Technical specs at a glance
| Method | Multiplex real-time PCR (4 tubes) |
| Analytes | 14 targets (CT, NG, HSV1/2, UU, UP, MH, MG, CA, GV, TV, GBS, HD, TP) |
| Specimens | Cervical swab, anorectal swab, urine, self-collected vaginal |
| Throughput | Batchable on common 96-well qPCR platforms |
| LOD (typical) | ≈10^2–10^3 copies/mL for CT in lab validation; real-world use may vary |
| Shelf life | ≈12–18 months at 2–8°C (check COA/IFU for exact date) |
| Controls | Internal control + external positive/negative recommended |
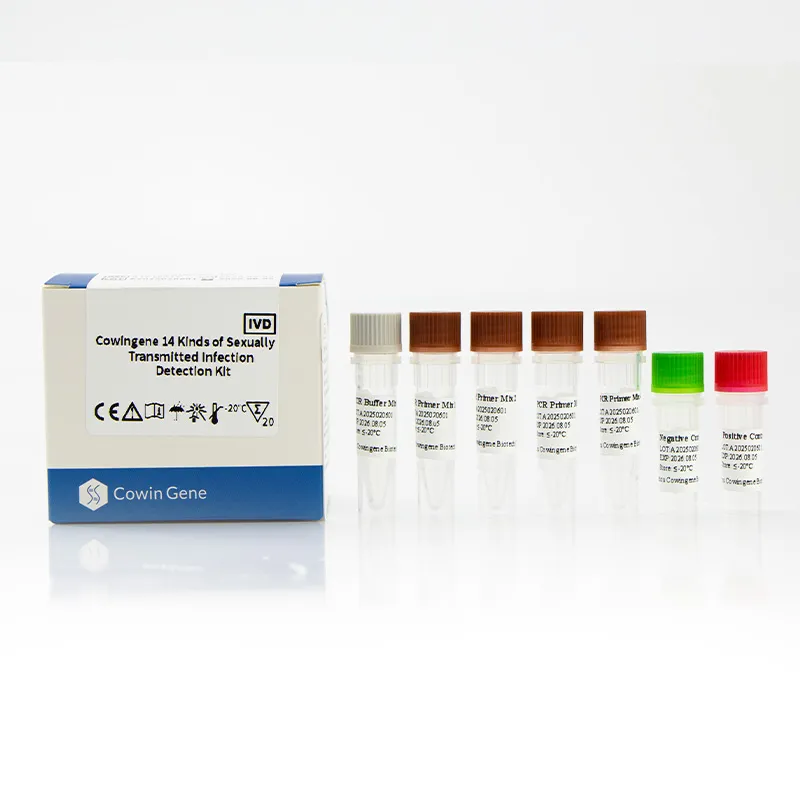
Process flow (materials, methods, standards)
- Materials: kit reagents (4-tube ready mixes), swab/urine collection devices, extraction kit (silica or magnetic), qPCR plates.
- Extraction: standard NAAT extraction; inhibit checks via internal control.
- Amplification: multiplex RT-PCR per IFU cycling; plate seals matter—learned that the hard way.
- QC: include NTC, positive controls every run; Westgard rules are your friend.
- Standards: follow CLSI MM06 and local verification protocols; document LOD and precision.
- Service life: store cold, avoid freeze–thaw; monitor control Ct drift over time.
Application scenarios
High-volume hospital labs, community sexual health clinics, NGO screening programs, and university health centers. Self-collection options reduce barriers for detection chlamydia trachomatis and partners in contact-tracing drives.
Vendor landscape (neutral snapshot)
| Vendor/Assay | Panel scope | Instrumentation | TAT |
|---|---|---|---|
| Cowingene 14 STI (Liquid) | 14 targets incl. chlamydia trachomatis, MG, TV | Open qPCR platforms | ≈2–3 h (batch) |
| Cepheid Xpert CT/NG | 2 targets (CT/NG) | GeneXpert | ≈60–90 min (cartridge) |
| Roche cobas CT/NG | 2 targets (CT/NG) | cobas systems | High-throughput |
Note: features are summarized from public materials; verify local regulatory status and IFUs.
Customization and integration
Labs often request custom report layouts (e.g., reflex macrolide resistance for MG) and LIS result mapping. Cowingene can provide documentation for verification, and—customers say—quick tech support from Taizhou.
Performance and feedback
Internal studies we’ve seen reported CT sensitivity ≈97–99% and specificity ≈98–100% versus a reference NAAT, with Ct consistency across matrix types. A regional clinic told us “one visit, one panel” cut callbacks by a third. It seems that batching helps control costs without sacrificing reliable detection chlamydia trachomatis.
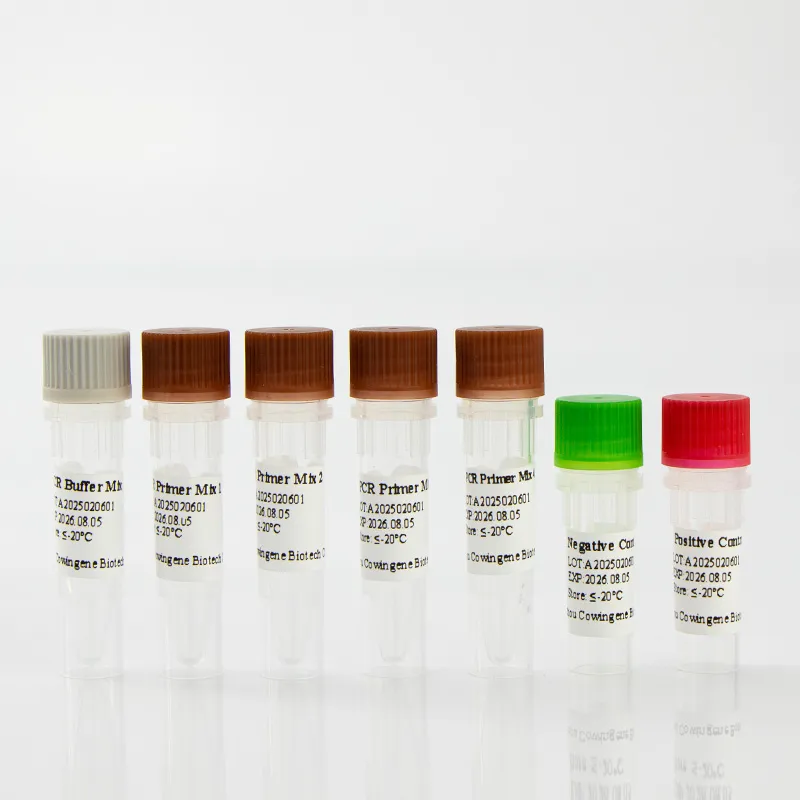
Compliance and documentation
Look for ISO 13485 QMS, risk management per ISO 14971, and—where applicable—IVD regulatory clearance. Labs should validate per CLSI and align with CDC/WHO screening guidance before go-live.
Closing note
If you’re upgrading from single-plex CT testing, a 14-analyte panel can streamline triage and treatment. Not perfect every day (what is?), but when the controls are tight and the workflow’s smooth, the numbers—and patients—benefit.
References
- CDC. Sexually Transmitted Infections Treatment Guidelines: Chlamydial Infections, 2021.
- WHO. Laboratory diagnosis of sexually transmitted infections, including use of NAATs, 2013 (and updates).
- CLSI MM06. Verification and Validation of Multiplex Nucleic Acid Assays, latest edition.
- ISO 13485:2016 Medical devices—Quality management systems; ISO 14971:2019 Risk management for medical devices.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







