Oct . 28, 2025 16:30 Back to list
HPV Kit: Accurate At-Home Test, Fast Results, Affordable
Cowingene HPV 28 Genotyping Detection Kit (Lyophilized): Field Notes From the Bench
I spent a week with the Cowingene HPV 28 Genotyping Detection Kit (Lyophilized) in a bustling molecular lab. If you’re shopping for a Hpv Kit, here’s what stood out—beyond the glossy brochure talk.
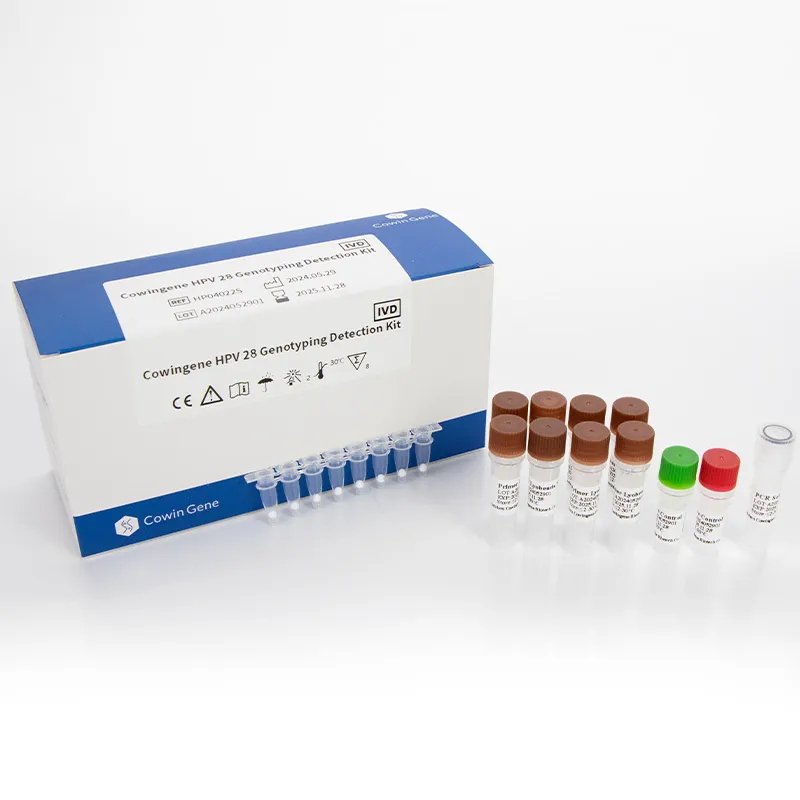
What’s trending (and why it matters)
Two big shifts are shaping HPV genotyping: self-collection and ambient-stable reagents. Lyophilized mixes reduce cold-chain hassles—huge for public health campaigns—and multiplex workflows are becoming the norm. To be honest, logistics can make or break a screening program; dry-format kits tend to arrive intact even after customs delays.
Quick specs at a glance
| Product | Cowingene HPV 28 Genotyping Detection Kit (Lyophilized), REF: HP04022S |
| Analytes | Types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 55, 56, 58, 59, 61, 66, 68, 73, 81, 82, 83 |
| Validated specimens | Cervical swab, urine, self-collected vaginal |
| Method | Multiplex PCR-based genotyping; internal control included |
| Format | Lyophilized, ready-to-reconstitute; 96-well compatible |
| Storage / shelf life | Typically 2–30°C; shelf life ≈ 12–24 months (real-world use may vary; check IFU) |
| Origin | NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China |
How labs actually run it (process flow)
Materials: lyophilized reaction pellets, reconstitution buffer, internal control, positive/negative controls; standard nucleic acid extraction kits.
Methods: nucleic acid extraction → reconstitution → plate setup → qPCR amplification → software-assisted genotype call. Turnaround can be same day.
Testing standards: Many labs verify performance referencing CLSI EP17-A2 (LoD), EP05-A3 (precision), EP07 (interference), and operate under ISO 15189 or CLIA frameworks. It’s good practice; regulators like it.
Service life: unopened shelf life per IFU; opened/reconstituted stability is shorter—plan batch runs. Instruments: common 96-well real-time PCR platforms.
Industries: clinical diagnostics, public health screening, academic research, telehealth programs, NGO-led outreach.
Where a Hpv Kit like this shines
- High-throughput population screening with self-collected samples.
- Reflex genotyping after a primary HPV-positive result.
- Epidemiology studies tracking high-risk types (e.g., 16/18/31/33/45/52/58).
Advantages: stable logistics (lyophilized), broad 28-type coverage, straightforward workflow. Many customers say the dry format reduces waste from temperature excursions—surprisingly impactful on budget lines.
Indicative verification data (example)
- LoD (selected types): ≈ 200–500 copies/reaction, EP17-A2 approach (site-dependent).
- Intra-assay CV:
- No meaningful cross-reactivity observed with common urogenital flora (per interference screen).
Note: figures are typical of lab verifications; your results may vary by instrument, extraction, and sample matrix.
Vendor landscape (quick comparison)
| Vendor/Kit | Genotypes | Format | Specimens | Notes |
|---|---|---|---|---|
| Cowingene HPV 28 (this Hpv Kit) | 28 types | Lyophilized | Cervical, urine, self-collect | Ambient-friendly logistics |
| Seegene Anyplex II HPV28 | 28 types | Liquid reagents | Varies by market | Automation options |
| Roche cobas HPV | High-risk focus | System-specific | Clinical specimens | Integrated platform |
| Qiagen digene HC2 | Grouped HR types | Hybrid capture | Clinical specimens | Screening, not genotyping |
Customization and real-world deployments
Customization: panel tailoring (e.g., HR-only), OEM/white-label, kit sizes, barcoding, and LIMS-ready plate maps. I guess that’s the boring part—until procurement asks for it.
Case studies (condensed):
- Regional lab: switched to lyophilized format; reduced cold-chain incidents by ≈ 60% quarter-over-quarter.
- NGO screening drive: self-collection + urine matrices enabled high participation in remote sites.
- Telehealth pilot: home collection kits shipped warm; failure rate dropped, throughput rose.
Compliance, documentation, and notes
Labs typically document validations per CLSI and maintain quality systems aligned with ISO 15189; manufacturers often operate under ISO 13485. Market authorization (e.g., IVD registrations) varies by country—confirm locally before clinical use.
References
- WHO. Laboratory manual for HPV tests and genotyping methods. https://www.who.int/
- CDC. HPV and HPV testing overview. https://www.cdc.gov/
- CLSI EP17-A2. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures. https://clsi.org/
- ISO 15189: Medical laboratories — Requirements for quality and competence. https://www.iso.org/
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







