Oct . 26, 2025 14:45 Back to list
MERS CoV PCR Kit: Fast, Sensitive, Reliable Results
What labs are really asking about right now: Mers Cov Pcr and practical multiplex options
If you run a respiratory diagnostics lab, you’ve probably noticed the same thing I have: demand is shifting toward multiplex RT‑PCR—fast triage for co-circulating targets, fewer hands-on steps, and data your clinicians can trust. And while Mers Cov Pcr remains a specialized need (mostly for travelers or outbreak investigations), multiplex kits for SARS‑CoV‑2 and influenza are doing the everyday heavy lifting. That’s exactly the niche where Cowingene’s liquid-format multiplex kit has been quietly gaining attention.
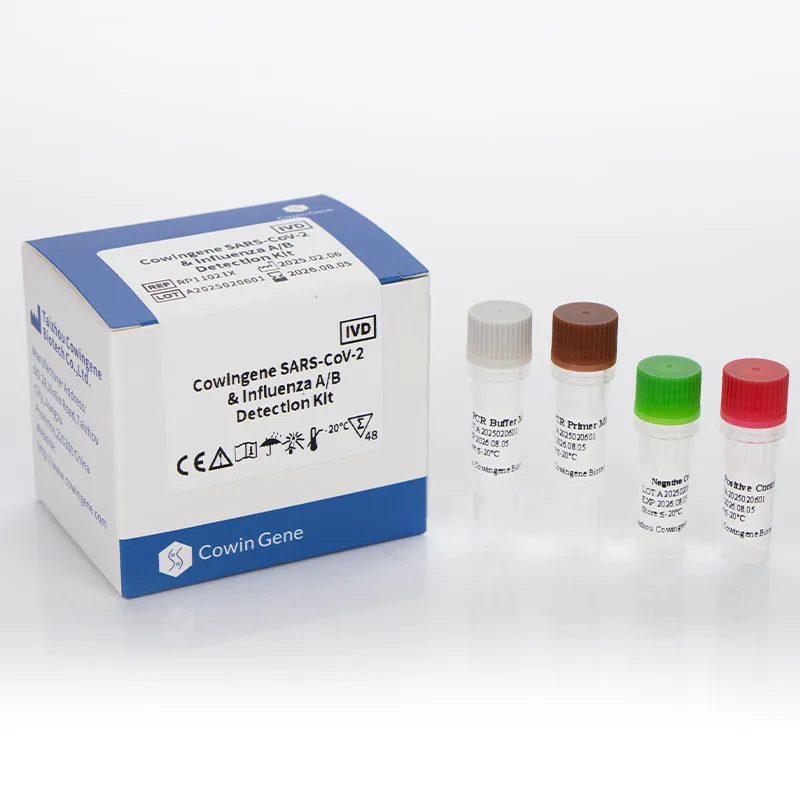
Product snapshot (and a quick reality check)
Cowingene SARS‑CoV‑2 & Influenza A/B Detection Kit (Liquid) is designed for multiplex detection of SARS‑CoV‑2, Flu A, and Flu B in a single tube. Important note: it’s not a Mers Cov Pcr assay. If you require MERS‑CoV, you’ll need a validated MERS‑specific rRT‑PCR following WHO/CDC protocols. That said, many customers say this Cowingene kit pairs well with a separate MERS assay during enhanced surveillance periods—less pipetting, fewer errors.
| Specification | Details (≈ / real‑world use may vary) |
|---|---|
| Product Name | Cowingene SARS‑CoV‑2 & Influenza A/B Detection Kit (Liquid) |
| REF | RP11021X |
| Analytes | 1 tube multiplex: SARS‑CoV‑2, Influenza A, Influenza B |
| Validated specimens | Nasopharyngeal swab/aspirate, Bronchoalveolar lavage, Throat swab |
| Format | Liquid master mix (multiplex) |
| Origin | NO.28, Xinlin Road, Taizhou, Jiangsu, China |
| Instrument compatibility | Common real‑time PCR cyclers; check IFU for channels and settings |
| Regulatory note | For professional use; regional registrations vary—confirm before clinical deployment |
Process flow (materials, methods, standards)
- Materials: validated swabs, VTM/UTM, RNA extraction kit (or validated extraction‑free protocol), calibrated qPCR system, controls.
- Methods: collect specimen → (optional) extraction → master mix prep (single tube) → thermal cycling → interpret Ct thresholds per IFU.
- Testing standards: adhere to WHO/CDC assay guidance, CLSI MM‑series for molecular methods, ISO 15189 for lab quality, and internal QC/lot verification.
- Service life: follow label; many liquid kits specify refrigerated stability; on‑board stability varies with cycler and workflow.
- Industries: hospital labs, public health networks, airport screening units, reference labs, and high‑throughput contract testing.
Why multiplex now?
Co‑circulation is the story of the season. Clinicians want one report that rules in/out COVID and flu quickly; administrators want fewer SKUs. In practice, a multiplex like this reduces pipetting steps and the dreaded “reflex testing” loop. For Mers Cov Pcr, labs typically layer in a WHO‑aligned rRT‑PCR only when the epi picture suggests it—travel history, clusters, or surveillance triggers.

Vendor comparison (pragmatic view)
| Vendor/Option | Targets | Workflow | Notes |
|---|---|---|---|
| Cowingene (this kit) | SARS‑CoV‑2, Flu A, Flu B | Single‑tube multiplex; standard qPCR | Good for routine respiratory panels; add separate MERS assay when indicated |
| Brand B Multiplex | SARS‑CoV‑2, Flu A/B, RSV | May require proprietary cycler | Broader panel; higher cost per test ≈ |
| LDT (in‑house) | Custom (incl. MERS possible) | Flexible but validation‑heavy | Great control; requires robust QA and ongoing maintenance |
Customization, feedback, small case notes
Some distributors tell me Cowingene can discuss OEM/private label and packaging lot sizes—handy if you’re juggling multiple testing sites. A mid‑size hospital network (Southeast Asia) reported fewer redraws after moving to a single‑tube multiplex, mainly due to streamlined prep. Another lab paired this kit with a WHO‑aligned Mers Cov Pcr protocol during a travel‑linked investigation—no instrument changes, just additional channels and controls. Customer sentiment skews positive on simplicity; turnaround depends on your extraction setup.
Data and compliance pointers
- Run external positive/negative controls each batch; monitor Ct drift across lots.
- For MERS, follow WHO/CDC target gene recommendations and confirmatory algorithms; LoD expectations in literature are typically low copy numbers per reaction, but verify locally.
- Document verification per CLSI and maintain ISO 15189 quality records; check manufacturer’s declarations (e.g., ISO 13485) before onboarding.
Disclaimer: This article is for professional audiences. The Cowingene kit described does not include a Mers Cov Pcr target; always consult the official IFU and your regulator before clinical use.
Authoritative citations
- WHO. Laboratory testing for Middle East respiratory syndrome coronavirus (MERS‑CoV). https://www.who.int/health-topics/middle-east-respiratory-syndrome-coronavirus-mers
- CDC. Real‑Time RT‑PCR Diagnostic Panel resources for respiratory viruses. https://www.cdc.gov/coronavirus/2019-ncov/lab/index.html
- CLSI. MM19 (Molecular Methods for Infectious Diseases) – guidance for verification/validation. https://clsi.org
- ISO 15189: Medical laboratories—Requirements for quality and competence. https://www.iso.org/standard/76677.html
- ECDC. Influenza testing and surveillance guidance. https://www.ecdc.europa.eu
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







