Oct . 20, 2025 14:50 Back to list
Rapid detection chlamydia trachomatis | Accurate PCR kit
Field Notes from the Lab: What’s Moving the Needle in STI Molecular Testing
If you work in a public health lab or a busy sexual health clinic, you already know the conversation has shifted from culture to rapid PCR—and not just for COVID. When teams talk about detection chlamydia trachomatis, the practical question is: who offers a reliable, multiplex kit that doesn’t punish your workflow or budget? I recently revisited Cowingene’s lyophilized CT/NG/TV kit and, to be honest, the real-world usability is what stood out.
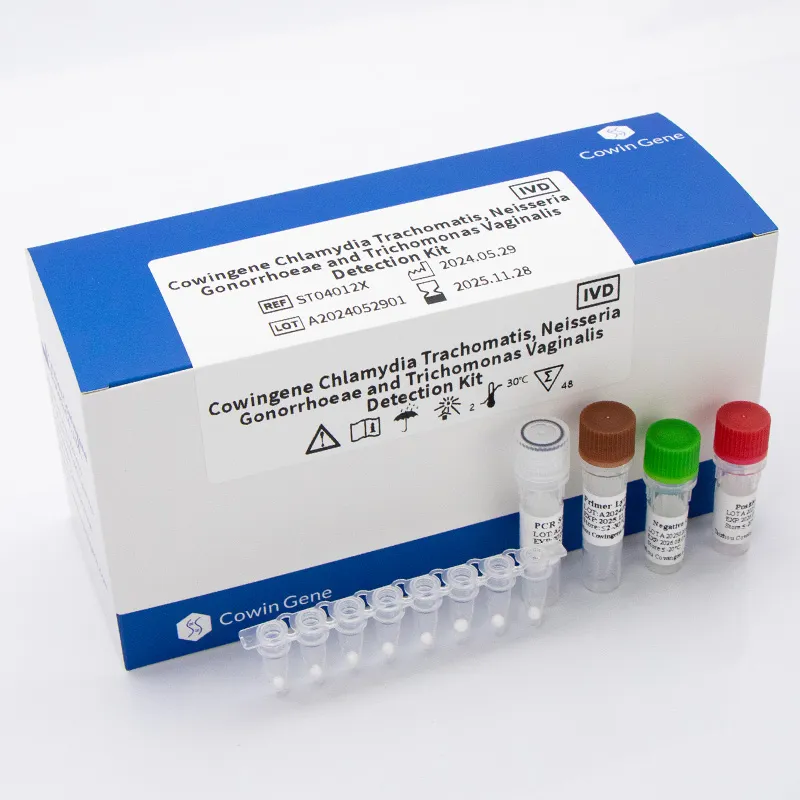
Product at a Glance
Cowingene Chlamydia Trachomatis, Neisseria Gonorrhoeae and Trichomonas Vaginalis Detection Kit (Lyophilized) — Origin: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China. REF: ST04012X. Validated specimens: cervical swab, anorectal swab, urine, self-collected vaginal. Analytes: CT, NG, TV. It’s a qPCR multiplex panel in a freeze-dried format, which, surprisingly, still feels underused in many labs given the storage and handling benefits.
Why Lyophilized Matters
- Operational simplicity: pre-aliquoted, less pipetting, fewer freeze–thaw cycles.
- Cold-chain relief: typically stable at 2–30°C; service life ≈ 18–24 months (real-world use may vary; follow IFU/lot docs).
- Consistency: reduced variability for multi-site rollouts—many customers say it cuts onboarding time.
Technical Specifications
| Parameter | Spec (≈ indicates typical) |
|---|---|
| Format | Lyophilized multiplex real-time PCR (hydrolysis probes) |
| Targets | Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Trichomonas vaginalis (TV), internal control |
| Specimens | Cervical, anorectal, urine, self-collected vaginal |
| LoD | Around 10²–10³ copies/mL per target (lot-dependent; verify per CLSI EP17-A2) |
| Run time | ≈ 60–90 min, instrument-dependent |
| Storage / Service life | 2–30°C; ≈ 18–24 months unopened (see COA/IFU) |
| Compatibility | Common qPCR platforms (e.g., ABI 7500, LightCycler, SLAN-type); check dye-channel mapping |
Process Flow (What It Looks Like in the Bench Book)
- Materials: lyophilized PCR tubes/plates, rehydration buffer, extraction kit or validated direct-lysis reagent, controls.
- Method: extract nucleic acids (or validated extraction-free approach), rehydrate lyophilized mix, add template, run qPCR.
- QC and standards: verify LoD and precision per CLSI EP17-A2 and EP05-A3; include positive/negative controls each run.
- Interpretation: system auto-calls CT/NG/TV; review amplification curves for borderline Ct values.
Vendor Comparison (real-world shorthand)
| Vendor/Kit | Targets | Format | TAT | Specimens | Notes |
|---|---|---|---|---|---|
| Cowingene CT/NG/TV (REF ST04012X) | CT, NG, TV | Lyophilized qPCR | ≈ 60–90 min | Cervical, anorectal, urine, self-collect vaginal | Lower cold-chain burden; flexible platforms |
| Vendor A Multiplex | CT, NG | Liquid master mix | ≈ 90 min | Urogenital only | Wider IVDR footprint; higher cold-chain costs |
| POCT B Cartridge | CT/NG | Closed cartridge | ≈ 30–45 min | Clinic swabs, urine | Great for decentral sites; higher per-test cost |
Use Cases and Feedback
- Sexual health clinics: same-day CT/NG/TV triage to cut loss-to-follow-up; detection chlamydia trachomatis alongside NG helps antimicrobial stewardship paths.
- University screening: batch-friendly; staff liked the simple rehydration step.
- Public health labs: multiplex reduces sample splits; one manager told me, “less juggling, fewer repeats.”
Compliance, Customization, and Data Points
Documentation typically aligns with ISO 13485 QMS; stability assessed per EN ISO 23640. Labs have validated with PPA/NPA figures in the high 90s for CT and NG (site-dependent), which tracks with CDC/WHO expectations for NAATs. If you need only detection chlamydia trachomatis or a different channel layout, ask about OEM/custom dye mapping and bulk lyophilized plates.
Testing Standards You’ll Likely Reference
- Verification: CLSI EP17-A2 (LoD), EP05-A3 (precision), EP12 (user evaluation), MM03/MM17 for molecular assays.
- Clinical guidance: CDC STD Treatment Guidelines; WHO guidance on CT/NG testing algorithms.
Final thought: the kit isn’t flashy—but that’s the point. In daily throughput, consistency beats spectacle. For many labs wrestling with multiplex adoption and cost control, this is a pragmatic move for detection chlamydia trachomatis with NG and TV on the same run.
References
- CDC. Sexually Transmitted Infections Treatment Guidelines, 2021. https://www.cdc.gov/std/treatment-guidelines/default.htm
- WHO. Laboratory diagnosis of sexually transmitted infections, including HIV. https://www.who.int/publications/i/item/9789241505840
- CLSI. EP17-A2: Protocols for Determination of Limits of Detection and Limits of Quantitation. https://clsi.org
- CLSI. EP05-A3: Evaluation of Precision of Quantitative Measurement Procedures. https://clsi.org
- EN ISO 23640:2015. IVD Medical Devices—Evaluation of Stability of IVD Reagents. https://www.iso.org
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







