Nov . 02, 2025 23:10 Back to list
Respiratory Panel Test for Fast, Accurate PCR Results
respiratory panel test for is a key solution in the healthcare industry, specifically within Medical Devices and Diagnostics and in vitro diagnosis. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
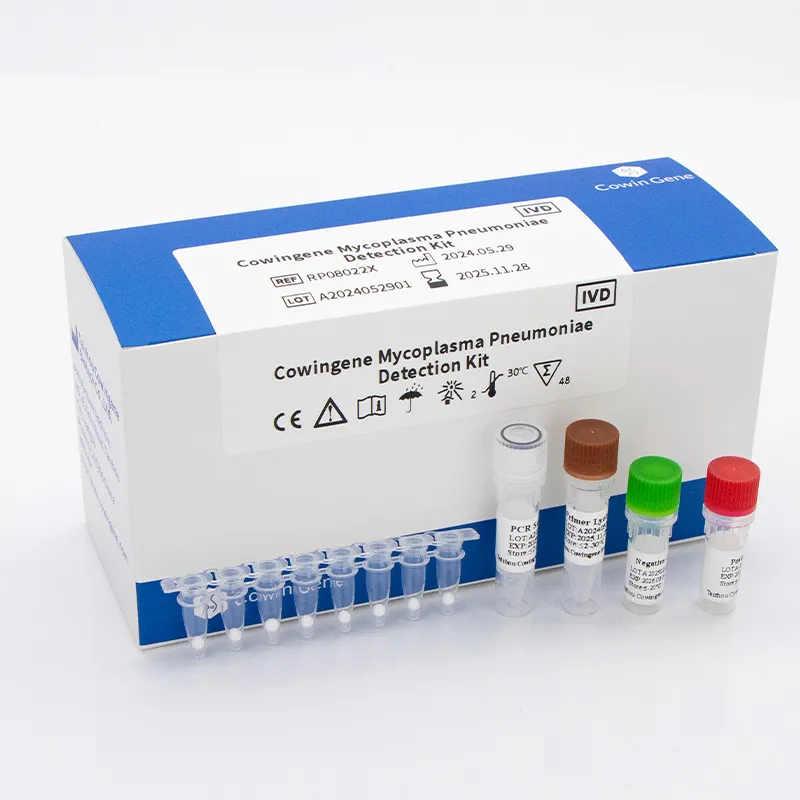
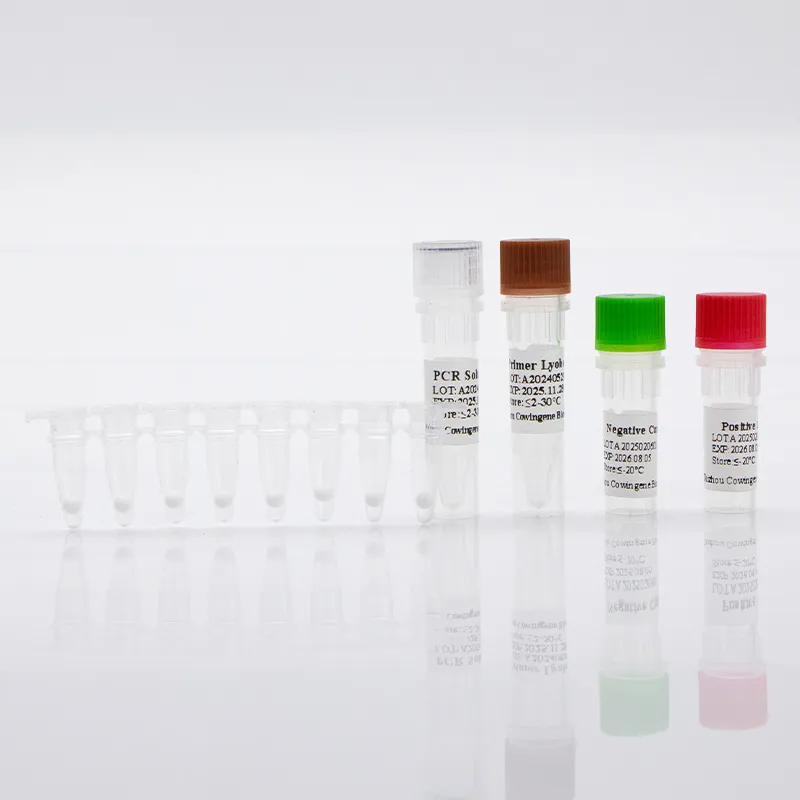
Table of Contents
- respiratory panel test for Overview
- Benefits & Use Cases of respiratory panel test for in in vitro diagnosis
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in healthcare
- Conclusion on respiratory panel test for from Taizhou Cowingene Biotech Co.,Ltd.
respiratory panel test for Overview
For laboratories and healthcare networks, a respiratory panel test for acute respiratory infections delivers timely, actionable insights by identifying specific pathogens from upper respiratory specimens. Taizhou Cowingene Biotech Co.,Ltd. offers a focused solution with its Mycoplasma pneumoniae Detection Kit (Lyophilized), designed for real-time PCR workflows. The kit supports respiratory pathogen panel strategies by enabling precise detection of an atypical pneumonia agent, helping labs complement broader multiplex menus or implement targeted reflex testing when syndromic screening flags bacterial etiology.
- Relevance: A respiratory panel test for M. pneumoniae aids differential diagnosis, antimicrobial stewardship, and surveillance programs in hospitals, reference labs, and public health settings.
- Technical background: Lyophilized RT-PCR reagents streamline shipping and storage, simplify setup, and are compatible with mainstream qPCR instruments. This format supports consistent Ct values and robust performance across runs typical of a respiratory pathogen panel PCR workflow.
- Manufacturer credibility: Taizhou Cowingene Biotech Co.,Ltd. is a trusted in vitro diagnostics supplier, engineering fit-for-purpose molecular assays that align with laboratory quality systems and throughput requirements.
Benefits & Use Cases of respiratory panel test for in in vitro diagnosis
B2B decision makers are aligning test menus to clinical pathways and operational KPIs. A respiratory panel test for Mycoplasma pneumoniae can be deployed as: 1) a standalone assay for atypical pneumonia cases, 2) a reflex test following a syndromic respiratory pathogen panel, or 3) part of a customized respiratory pathogen panel pcr workflow when targeted panels are preferred over large multiplexes. The lyophilized configuration minimizes pipetting steps, supporting reproducibility and faster hands-on times for medium-to-high-throughput labs.
- Applications: Hospital diagnostic labs, urgent care networks, and third-party reference labs use it to clarify atypical bacterial involvement in respiratory infections and to guide isolation policies and therapy decisions.
- Advantages: Lyophilized stability, compatibility with standard qPCR platforms, and clear interpretation workflows enable reliable integration into existing respiratory pathogen panel processes without extensive retraining.
- Expertise: Taizhou Cowingene Biotech Co.,Ltd. brings molecular design, manufacturing consistency, and technical support that help labs validate and scale their respiratory testing portfolios with confidence.
Cost, Maintenance & User Experience
Total cost of ownership matters as testing volumes surge seasonally. Lyophilized reagents can reduce cold-chain dependence, shipping expenses, and storage constraints—delivering tangible savings across the fiscal year. A respiratory panel test for M. pneumoniae that is easy to set up and robust across instrument platforms lowers onboarding time and minimizes repeat runs, supporting favorable ROI through operational efficiency and reduced waste. These factors help labs meet turnaround time goals while preserving margins.
- Durability and workflow: The kit’s ready-to-use format streamlines QC and routine maintenance by minimizing reagent prep steps, which can reduce variability and hands-on labor.
- User feedback: Medical Devices and Diagnostics customers value consistent performance, clear protocols, and responsive technical support from Taizhou Cowingene Biotech Co.,Ltd., citing smoother validations and reliable day-to-day runs in respiratory pathogen panel PCR environments.
Sustainability & Market Trends in healthcare
The respiratory diagnostics market continues to evolve toward faster molecular workflows, flexible menus, and decentralized testing. Sustainability is increasingly central: reducing cold-chain logistics, packaging volume, and consumable waste are meaningful levers for lowering a lab’s environmental footprint. A respiratory panel test for targeted pathogens, delivered in a lyophilized format, aligns with these goals while maintaining analytical performance. At the same time, labs must navigate quality standards, data integrity, and post-market vigilance to stay compliant.
- Regulatory and growth context: As demand for rapid, reliable respiratory testing remains high, platforms and assays that integrate seamlessly with LIS/middleware and quality management systems are best placed to scale responsibly.
- Forward-thinking partner: Taizhou Cowingene Biotech Co.,Ltd. invests in sustainable product design and customer-centric support, helping labs advance both operational resilience and environmental stewardship across the respiratory pathogen panel continuum.
Conclusion on respiratory panel test for from Taizhou Cowingene Biotech Co.,Ltd.
A well-designed respiratory panel test for Mycoplasma pneumoniae strengthens diagnostic clarity, supports antimicrobial stewardship, and fits seamlessly into respiratory pathogen panel PCR workflows. Taizhou Cowingene Biotech Co.,Ltd. delivers dependable, lyophilized PCR solutions that help labs optimize cost, throughput, and sustainability without compromising quality. Ready to upgrade your respiratory testing workflow? Contact us: email: info@cowingene.com — Visit our website: https://www.cowingene.com
Related PRODUCTS
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
NewsNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
NewsNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
NewsNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
NewsNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
NewsNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
NewsNov.04,2025







