Nov . 03, 2025 00:35 Back to list
Respiratory Pathogen Panel: Rapid, Accurate Multiplex Tests
Respiratory Pathogen Panel is a key solution in the healthcare industry, specifically within medical device and in vitro diagnosis. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
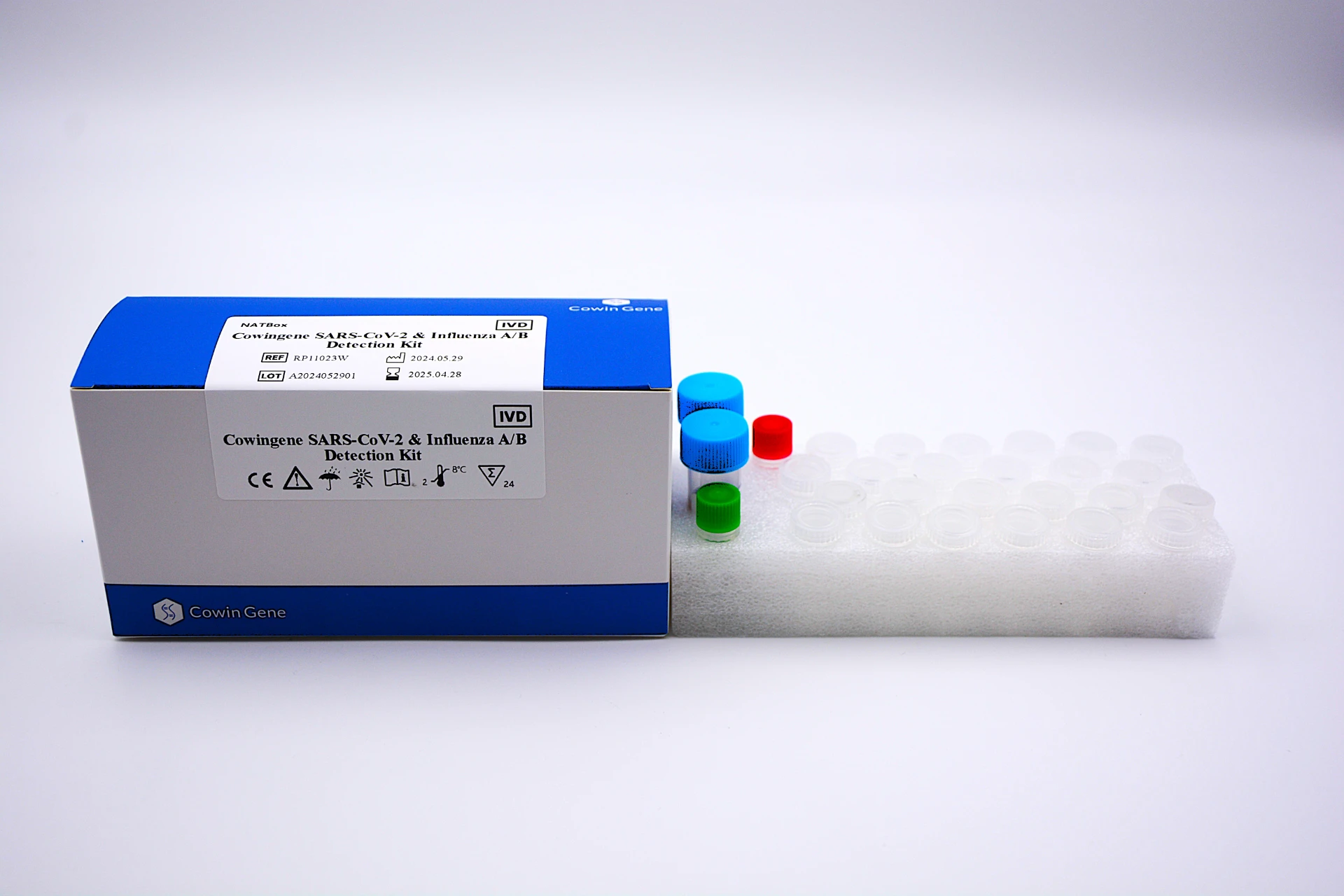
Table of Contents
- Respiratory Pathogen Panel Overview
- Benefits & Use Cases of Respiratory Pathogen Panel in in vitro diagnosis
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in healthcare
- Conclusion on Respiratory Pathogen Panel from Taizhou Cowingene Biotech Co.,Ltd.
Respiratory Pathogen Panel Overview
In diagnostics, a Respiratory Pathogen Panel (RPP) is a multiplex assay that detects and differentiates multiple respiratory agents in a single run—streamlining triage, optimizing isolation decisions, and guiding therapy. For B2B decision makers across the in vitro diagnosis value chain, RPPs reduce diagnostic ambiguity in peak seasons and support laboratory throughput. The Taizhou Cowingene Biotech Co.,Ltd. solution focuses on high-prevalence targets such as SARS‑CoV‑2 and Influenza A, offering multiplex real-time PCR detection in one workflow, with internal controls and standardized reagents for consistent performance.
- Relevance: Consolidates multiple singleplex assays into one Respiratory Pathogen Panel to accelerate reporting and improve resource utilization in clinical laboratories.
- Technical background: Multiplex RT‑qPCR chemistry designed for nasopharyngeal/oropharyngeal swabs; compatible with mainstream qPCR thermocyclers and standard nucleic acid extraction methods; includes positive/negative controls for quality monitoring.
- Manufacturer credibility: Taizhou Cowingene Biotech Co.,Ltd. provides durable, high-performance IVD consumables backed by robust manufacturing practices and professional technical support for healthcare and medical device partners.
Benefits & Use Cases of Respiratory Pathogen Panel in in vitro diagnosis
In IVD workflows, the Respiratory Pathogen Panel enables rapid differentiation of clinically overlapping infections. Laboratories deploy the respiratory panel test for high-acuity emergency departments, seasonal influenza waves, outpatient clinics, and surveillance programs. By consolidating SARS‑CoV‑2 and Influenza A detection into a single tube, Taizhou Cowingene Biotech Co.,Ltd. helps reduce repeat sampling and unnecessary reflex testing, improving turnaround for critical cases and optimizing staff utilization.
- Applications: Hospital labs, independent reference laboratories, and public health units that require reliable, scalable respiratory pathogen testing during peak demand.
- Competitive advantages: Single‑run multiplexing, streamlined QC with built‑in controls, broad instrument compatibility, and standardized protocols that simplify onboarding and reduce variability across sites.
- Sector expertise: As an experienced IVD supplier, Taizhou Cowingene supports method verification, workflow design, and training resources—helping B2B partners operationalize RPPs with confidence.
Cost, Maintenance & User Experience
Total cost of ownership for an RPP hinges on reagent price, instrument utilization, labor, and repeat-test rates. A multiplex Respiratory Pathogen Panel can lower per-report costs by reducing consumables, hands-on time, and second-line testing. Because Taizhou Cowingene’s panel works on widely used qPCR platforms, labs can leverage existing capital assets—minimizing new equipment purchases and shortening implementation timelines.
- Durability & ROI: Standardized kit components and stable chemistry support consistent runs across shifts, while fewer repeat tests improve reagent yield and throughput—key drivers of ROI during seasonal surges.
- User experience: Clear IFU, integrated controls, and compatibility with automated extraction reduce training time and error risk. Procurement teams appreciate predictable supply and technical support continuity from a focused IVD manufacturer.
Sustainability & Market Trends in healthcare
The respiratory pathogen testing kits market continues to evolve with demands for higher multiplexing, faster turnaround, and scalable logistics. Regulatory frameworks such as FDA, CE, and IVDR requirements encourage analytical transparency, traceability, and robust post‑market surveillance. Forward-looking IVD organizations prioritize eco‑conscious packaging, optimized cold‑chain shipping, and reduced plastic per test—without compromising quality and safety.
- Sustainability: Taizhou Cowingene Biotech Co.,Ltd. emphasizes efficient packaging, streamlined logistics, and long-run product reliability to help partners cut waste and shipment frequency.
- Market outlook: Persistent co-circulation of respiratory viruses sustains demand for reliable RPPs. Labs are prioritizing panels that integrate seamlessly into LIS/middleware and standard qPCR platforms—areas where Cowingene’s pragmatic, compatibility-first approach delivers practical value.
Conclusion on Respiratory Pathogen Panel from Taizhou Cowingene Biotech Co.,Ltd.
For healthcare organizations and medical device partners, a high-confidence Respiratory Pathogen Panel enhances diagnostic clarity, accelerates reporting, and supports operational resilience. Taizhou Cowingene Biotech Co.,Ltd. combines technical rigor with dependable supply to help labs deploy a respiratory panel test for high-demand seasons and routine care alike. Explore the product page to learn how our solution fits your workflow and platforms.
- Contact us: email: info@cowingene.com
- Visit our website: https://www.cowingene.com
Related PRODUCTS
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
NewsNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
NewsNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
NewsNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
NewsNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
NewsNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
NewsNov.04,2025







