Oct . 05, 2025 22:20 Back to list
Cowingene HPV 28 Genotyping Detection Kit-PCR Technology|NIST Certified
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) is a cutting-edge molecular diagnostic tool designed for the rapid and precise identification of 28 high-risk human papillomavirus (HPV) genotypes. Developed by Taizhou Cowingene Biotech Co., Ltd., this kit leverages advanced polymerase chain reaction (PCR) technology to deliver reliable results in clinical and research settings. This article explores the product’s features, advantages, technical specifications, application scenarios, and the company’s expertise in molecular diagnostics.
Product Features
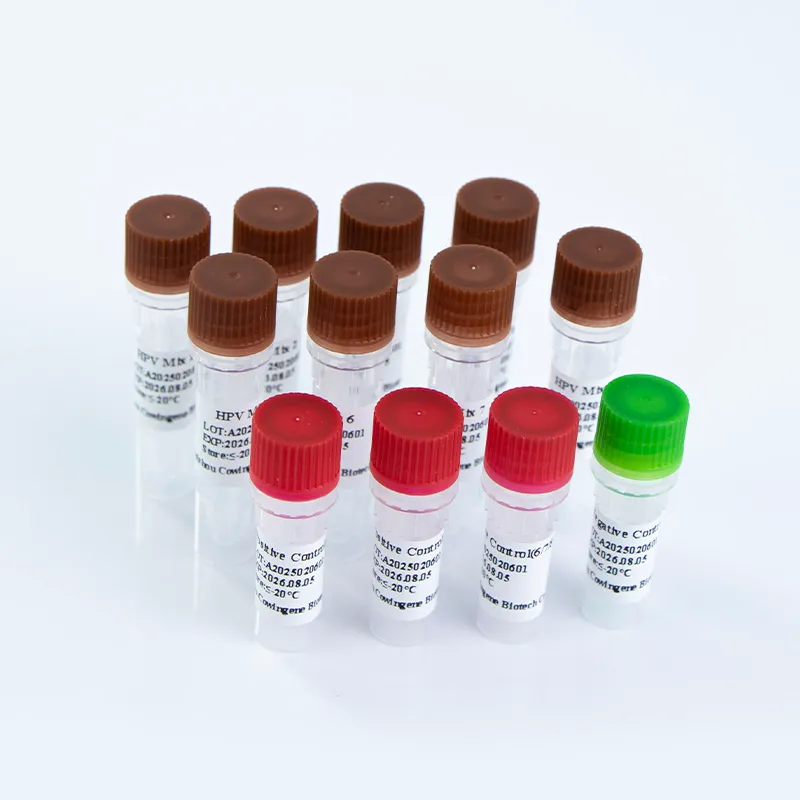
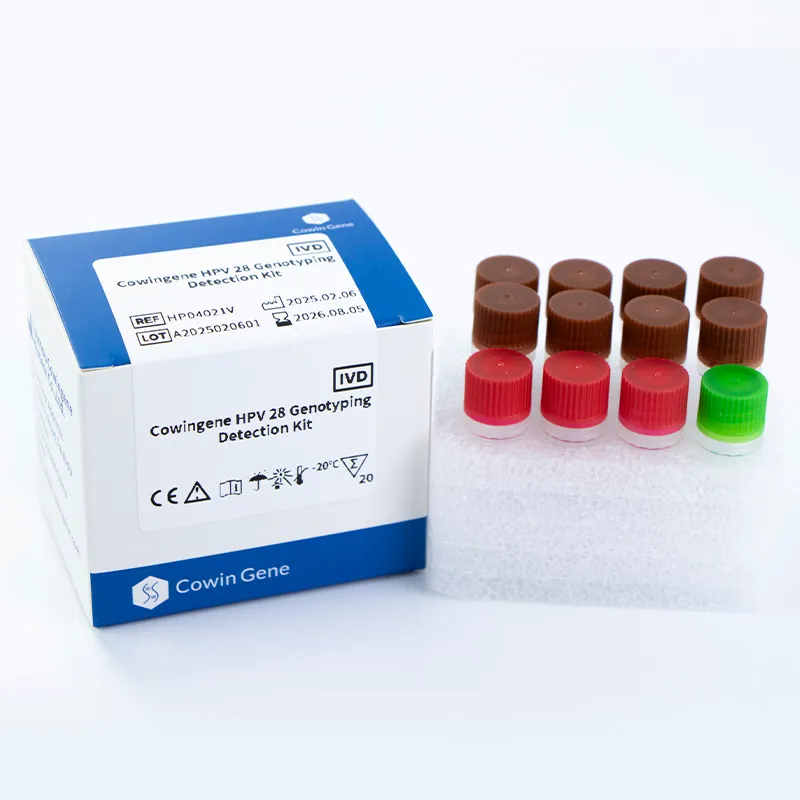
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) is engineered to detect 28 high-risk HPV genotypes, including 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 55, 56, 58, 59, 61, 66, 68, 73, 81, 82, 83, and 85. This comprehensive coverage ensures the detection of the most clinically relevant HPV subtypes associated with cervical cancer and other gynecological conditions. The kit utilizes a liquid-based system, which enhances sample stability and reduces the risk of cross-contamination during testing.
Read More About human papilloma virus HPV PCR
One of the standout features of this kit is its ability to process a wide range of validated specimen types, including cervical swabs, urine, and self-collected vaginal samples. This versatility makes it an ideal solution for both clinical laboratories and point-of-care settings. The kit’s design also emphasizes user-friendliness, with a streamlined workflow that minimizes the need for specialized training. This makes it accessible to a broad range of healthcare professionals, from laboratory technicians to clinicians.
Advantages of the Cowingene HPV 28 Genotyping Detection Kit
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) offers several key advantages that set it apart from traditional HPV detection methods. First, its high sensitivity and specificity ensure accurate identification of HPV genotypes, even in low viral load samples. This is critical for early detection and effective treatment planning. Second, the kit’s rapid turnaround time allows for timely diagnosis, which is essential in managing cervical cancer risk.
Read More About human papillomavirus PCR
Another significant advantage is the kit’s compatibility with automated and semi-automated PCR systems, which reduces manual handling and minimizes the risk of human error. This feature is particularly beneficial for high-throughput laboratories that require consistent and reproducible results. Additionally, the kit’s liquid-based format ensures that samples remain stable during storage and transportation, reducing the likelihood of sample degradation.
Technical Specifications
| Parameter | Details |
|---|---|
| Tested HPV Genotypes | 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 55, 56, 58, 59, 61, 66, 68, 73, 81, 82, 83, 85 |
| Sample Types | Cervical swab, Urine, Self-collected vaginal |
| Technology | PCR-based genotyping |
| Kit Components | 8 tubes, reagents, controls, and a detailed protocol |
| Storage Conditions | -20°C for long-term storage; 4°C for short-term use |
| Turnaround Time | Less than 4 hours |
The technical specifications of the Cowingene HPV 28 Genotyping Detection Kit (Liquid) highlight its precision and efficiency. The kit’s ability to detect 28 HPV genotypes in a single test reduces the need for multiple assays, saving time and resources. The inclusion of 8 tubes ensures that multiple samples can be processed simultaneously, enhancing laboratory productivity. Furthermore, the kit’s reagents are optimized for high amplification efficiency, which is crucial for accurate genotyping.
Application Scenarios
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) is designed for a variety of clinical and research applications. In clinical settings, it is used for cervical cancer screening, where early detection of high-risk HPV genotypes can significantly improve patient outcomes. The kit’s ability to process self-collected vaginal samples makes it particularly useful in resource-limited areas, where access to traditional cervical swab collection methods may be limited.
Read More About human papilloma virus HPV PCR
In research environments, the kit is employed to study the prevalence and distribution of HPV genotypes in different populations. This data is invaluable for epidemiological studies and the development of targeted vaccines. Additionally, the kit’s compatibility with automated PCR systems makes it suitable for large-scale screening programs, such as those implemented by public health organizations.
Company Background: Taizhou Cowingene Biotech Co., Ltd.
Taizhou Cowingene Biotech Co., Ltd. is a leading biotechnology company specializing in molecular diagnostics and infectious disease testing. Based in Taizhou, China, the company has built a reputation for developing innovative solutions that address critical healthcare challenges. With a focus on research and development, Cowingene has established itself as a trusted provider of high-quality diagnostic products worldwide.
Over the years, Cowingene has invested heavily in advanced technologies, including PCR-based assays and next-generation sequencing, to ensure the accuracy and reliability of its products. The company’s commitment to quality is reflected in its adherence to international standards for diagnostic testing. This dedication to excellence has earned Cowingene a strong presence in both domestic and global markets.
Compliance with NIST Standards
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) is designed to meet the rigorous standards set by the National Institute of Standards and Technology (NIST). NIST plays a critical role in developing measurement standards that ensure the accuracy and consistency of diagnostic tools. By aligning with these standards, Cowingene ensures that its products deliver reliable results that can be trusted by healthcare professionals and researchers alike.
According to NIST’s guidelines, diagnostic tests must demonstrate high specificity and sensitivity to minimize false positives and negatives. The Cowingene kit’s ability to detect 28 HPV genotypes with minimal cross-reactivity is a testament to its compliance with these standards. Furthermore, the kit’s standardized protocols and validated reagents align with NIST’s emphasis on reproducibility and traceability in diagnostic testing.
Conclusion
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) represents a significant advancement in HPV detection technology. Its comprehensive coverage of 28 high-risk genotypes, coupled with its user-friendly design and rapid results, makes it an indispensable tool for both clinical and research applications. With its adherence to NIST standards and the expertise of Taizhou Cowingene Biotech Co., Ltd., this kit sets a new benchmark for accuracy and reliability in molecular diagnostics.
References
NIST. (n.d.). National Institute of Standards and Technology. Retrieved from https://www.nist.gov/
U.S. Department of Commerce. (n.d.). U.S. Department of Commerce. Retrieved from https://www.commerce.gov/
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025