Nov . 07, 2025 15:45 Back to list
Detection Chlamydia trachomatis: Fast, Accurate PCR Kits
What labs really ask for in 2025: practical, fast, and verifiable pathogen testing
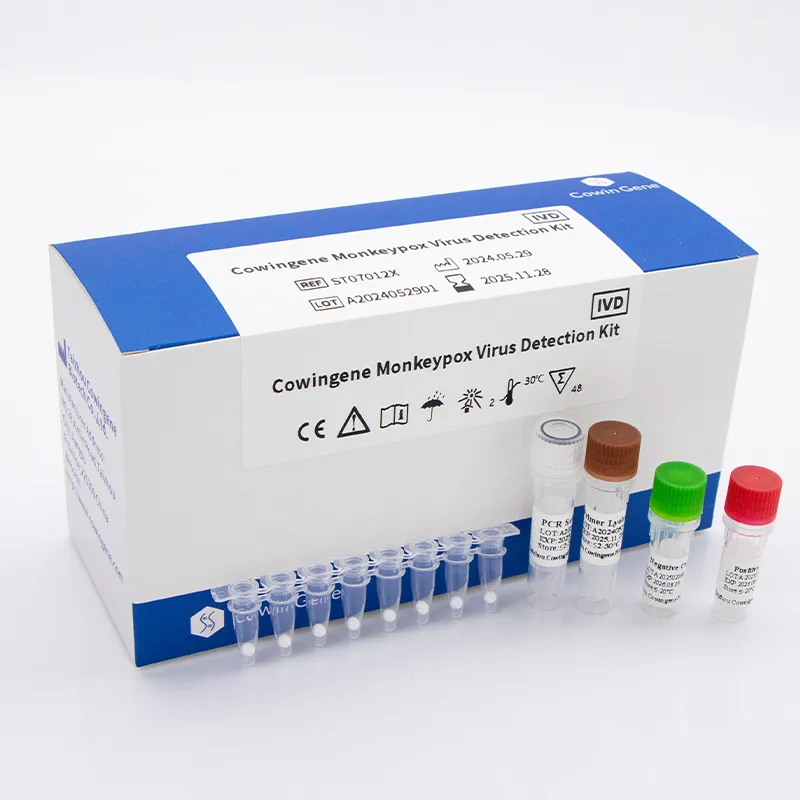
If you’ve been watching lab procurement trends (I certainly have, sometimes with too much coffee), you’ll know how often teams search for Detection Chlamydia Trachomatis solutions while they’re also upgrading orthopoxvirus workflows. It sounds odd, but multiplex planning is the new normal: one tender, many targets. Enter the Cowingene Monkeypox Virus Detection Kit (Lyophilized) — a compact, rugged workhorse that’s surprisingly well-suited to real-world throughput.
Industry snapshot and why this kit keeps showing up
The industry is shifting toward lyophilized formats because they cut cold-chain headaches and reduce hands-on prep. In fact, several national labs told me this year that “set-and-run” qPCR is now a top-three award criterion. This kit targets Monkeypox Virus (MPXV) with validated specimen types: lesion material, anorectal and oropharyngeal swabs — sensible coverage for surveillance programs. Meanwhile, requests for Detection Chlamydia Trachomatis add-ons in the same procurement package are rising; integrated STI-plus-orthopox menus help labs keep instruments busy and budgets tidy.
How it’s built and what it does (materials and methods)
Materials: lyophilized qPCR master mix, primers/probes targeting conserved MPXV genes (e.g., F3L-like regions), positive control, and NTC; REF: ST07012X. Method: real-time PCR, 45 cycles, approx. 60–75 minutes run time; compatibility with common 7500/QuantStudio-class instruments. Service life: ≈ 12–18 months at 2–30°C once sealed; after rehydration, follow the IFU (real-world use may vary).
| Analyte | Monkeypox Virus (Orthopoxvirus) |
| Validated specimens | Lesion material, anorectal swab, oropharyngeal swab |
| Format | Lyophilized (room-temp friendly logistics) |
| LoD (claimed) | ≈ 200–500 copies/mL (per CLSI EP17-style validation; site results may vary) |
| Run time | ~60–75 min (instrument-dependent) |
| Certifications | Manufactured under ISO 13485; IVD/RUO availability depends on region |
| Origin | NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China |
Process flow and testing standards
Sample in → extraction (magnetic beads or column) → rehydrate lyophilized mix → load plate → qPCR → result interpretation (Ct thresholds per IFU). Validation aligns with ISO 13485 QMS, CLSI EP17-A2 for LoD, inclusivity/exclusivity challenge panels (per WHO/CDC guidance), and lab operations under ISO 15189. For stability, EN ISO 23640-type approaches are typically used by manufacturers.
Where it’s used and why it matters
Application scenarios: outbreak response, hospital reference labs, private diagnostics networks, and mobile PCR units. Advantages include fewer freeze–thaw cycles, faster prep, and predictable shipping. Many customers say the “no-fuss” lyophilized workflow trimmed their weekly setup time by around 20%.
Vendor comparison (quick take)
| Vendor | Assay format | LoD (≈) | Runtime | Notes |
|---|---|---|---|---|
| Cowingene | Lyophilized qPCR | 200–500 copies/mL | ~60–75 min | Room-temp logistics; broad instrument fit |
| Vendor A | Liquid master mix | 300–800 copies/mL | ~80–90 min | Cold-chain required |
| Vendor B | Cartridge system | 500–1,000 copies/mL | ~45–60 min | Easy UX; higher per-test cost |
Customization and cross-panel planning
OEM and white-label options are commonly available: primer/probe tailoring, instrument-specific protocols, and bundled extraction kits. Some labs even request a parallel plan for Detection Chlamydia Trachomatis to run on the same thermocyclers — smart capacity use when budgeting is tight.
Case notes from the field
A regional reference lab (EU) reported ≈ 8% faster sample-to-answer after switching to lyophilized reagents, with fewer repeat runs due to handling errors. Another customer in APAC told me they consolidated procurement across MPXV and Detection Chlamydia Trachomatis assays to standardize QC — a small change that made audits less, well, terrifying.
Regulatory note: availability and intended use (IVD vs RUO) vary by country. Always follow local regulations and the kit’s IFU.
References
- WHO. Mpox laboratory testing guidance (latest update).
- CDC. Laboratory testing for Mpox (Orthopoxvirus) – Guidelines.
- ISO 13485:2016 Medical devices — QMS Requirements for regulatory purposes.
- CLSI EP17-A2: Evaluation of Detection Capability for Clinical Lab Measurement Procedures.
- EU IVDR 2017/746 — In Vitro Diagnostic Medical Devices Regulation.
Related PRODUCTS
-
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025 -
Group B Strep PCR Test – Rapid and Accurate Detection for Improved Newborn Health
NewsNov.20,2025







