Oct . 16, 2025 13:45 Back to list
Detection Chlamydia trachomatis PCR Kit | Fast, Sensitive
detection chlamydia trachomatis,trachomatis pcr,pcr c trachomatis is a key solution in the biotechnology industry, specifically within in vitro diagnosis and Molecular Diagnostics. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
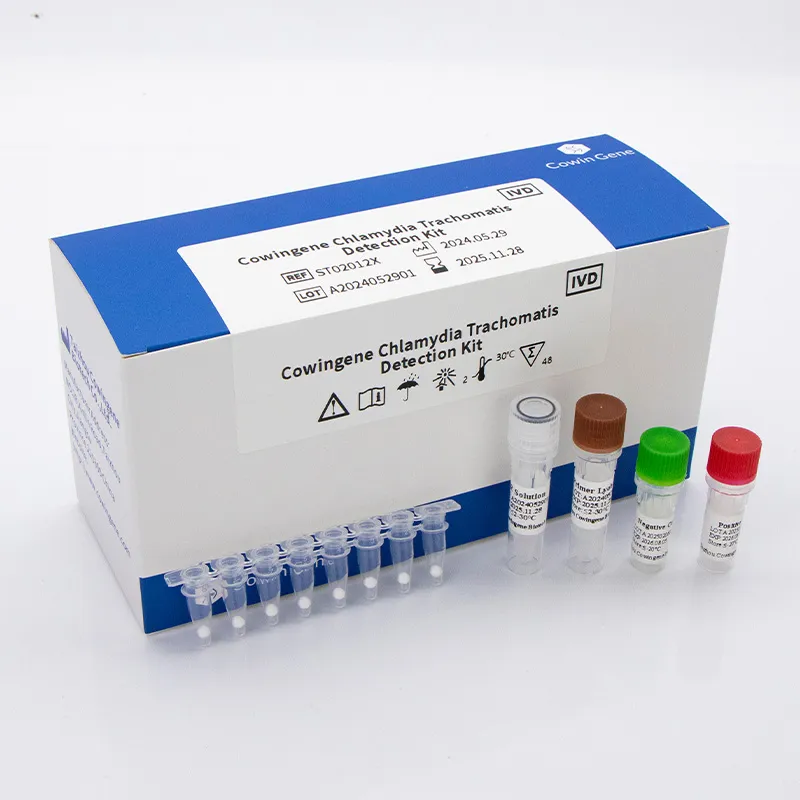
Table of Contents
- detection chlamydia trachomatis,trachomatis pcr,pcr c trachomatis Overview
- Benefits & Use Cases of detection chlamydia trachomatis,trachomatis pcr,pcr c trachomatis in Molecular Diagnostics
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in biotechnology
- Conclusion on detection chlamydia trachomatis,trachomatis pcr,pcr c trachomatis from Taizhou Cowingene Biotech Co.,Ltd.
detection chlamydia trachomatis,trachomatis pcr,pcr c trachomatis Overview
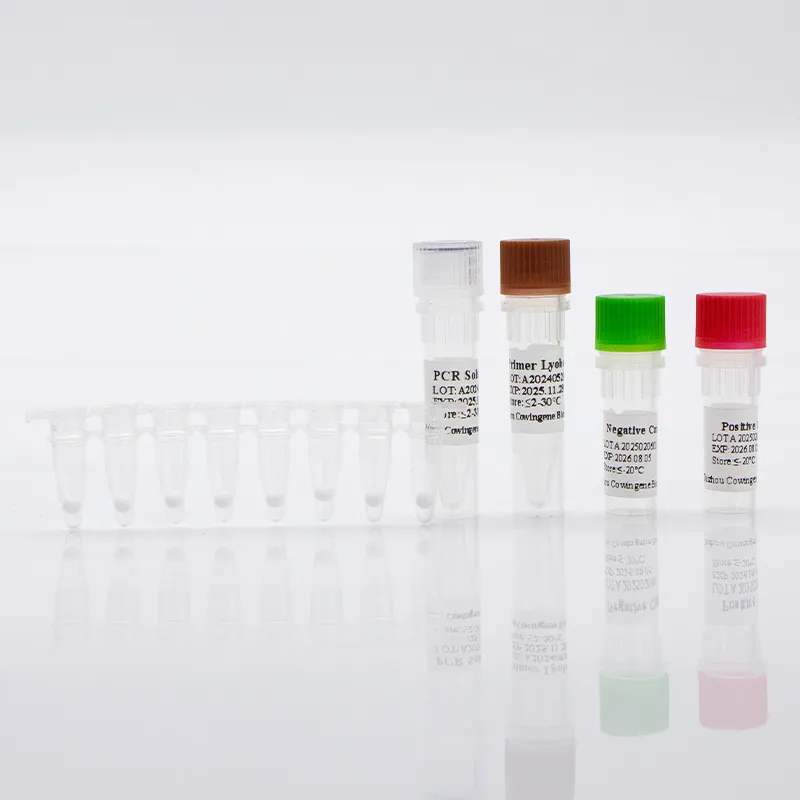
Chlamydia trachomatis remains one of the most commonly tested pathogens in molecular diagnostics, and laboratories increasingly rely on real-time PCR workflows to ensure rapid, accurate results. The Taizhou Cowingene Biotech Co.,Ltd. Chlamydia trachomatis Detection Kit is designed for qualitative nucleic acid amplification and detection workflows, helping laboratories standardize trachomatis PCR procedures from sample to result. Built for compatibility with mainstream real-time PCR instruments, the assay supports routine testing needs across reference labs, hospitals, and high-throughput networks.
Technically, the kit is optimized for robust amplification of C. trachomatis DNA using real-time PCR chemistry, supporting stringent quality control with positive/negative controls and internal process verification. Typical sample inputs include urogenital swabs and urine specimens, following validated nucleic acid extraction protocols. The streamlined reagent preparation, clear cycling parameters, and straightforward interpretation criteria enable efficient turnaround within standard qPCR run times. In a representative deployment, a regional diagnostics provider consolidated multiple legacy methods into a single, standardized trachomatis PCR workflow—reducing repeat rates and improving cross-site data consistency. With reliable manufacturing and stringent lot-to-lot traceability, Taizhou Cowingene Biotech Co.,Ltd. delivers dependable performance to support routine screening and confirmatory testing at scale.
Benefits & Use Cases of detection chlamydia trachomatis,trachomatis pcr,pcr c trachomatis in Molecular Diagnostics
For B2B decision makers, the primary value of trachomatis PCR is operational reliability, from consistent analytical performance to seamless integration with existing platforms. The kit’s design supports routine STI screening programs, reflex confirmatory testing, and surveillance initiatives where high sensitivity and specificity are essential to mitigate false results and repeat workflows. Its compatibility with mainstream extraction systems and qPCR platforms reduces validation burden and accelerates deployment across networks.
Key advantages include optimized assay robustness, intuitive workflows that minimize hands-on time, and comprehensive controls to support trustworthy result interpretation. The flexibility to implement pcr c trachomatis testing as a standalone assay—or as part of a broader STI testing strategy—makes it an effective tool for laboratories seeking scalability. Taizhou Cowingene Biotech Co.,Ltd. brings deep molecular diagnostics expertise, backed by rigorous quality management and application support, empowering labs to improve throughput without compromising accuracy. Whether your priority is standardizing detection chlamydia trachomatis protocols across multiple sites or enhancing capacity during peak demand, the solution is engineered to fit modern lab operations.
Cost, Maintenance & User Experience
Total cost of ownership in molecular diagnostics hinges on more than list price; it includes labor, repeat testing, instrument utilization, and quality assurance. By simplifying reagent setup and streamlining the trachomatis PCR workflow, Taizhou Cowingene Biotech Co.,Ltd.’s kit helps reduce hands-on time and consumable usage—lowering cost per reportable result. Standardized protocols shorten training cycles and reduce operator-dependent variability, which can significantly cut repeat rates and associated costs.
Customers in the in vitro diagnosis sector report smoother onboarding, consistent lot performance, and dependable supply continuity—key to maintaining stable turnaround times. The kit’s durable packaging and clear IFU support routine lab maintenance practices, while compatibility with existing qPCR cyclers preserves prior equipment investments. From an ROI standpoint, labs benefit from predictable performance, fewer workflow interruptions, and improved throughput, especially when integrated with automated extraction platforms. For decision makers, these efficiencies translate into scalable capacity, reliable TAT, and measurable operational savings across multi-site networks.
Sustainability & Market Trends in biotechnology
The molecular diagnostics market for STI testing continues to expand, driven by screening initiatives, public health surveillance, and a sustained shift to nucleic acid amplification tests. Evolving regulatory frameworks—such as strengthened quality requirements and documentation under international standards—are elevating expectations for traceability, validation support, and post-market surveillance. Vendors that can provide robust technical files, stable supply chains, and responsive customer service gain a competitive edge.
Taizhou Cowingene Biotech Co.,Ltd. aligns with these trends by emphasizing quality manufacturing, data-driven QC, and continuous improvement. The company also advances sustainability by optimizing packaging, consolidating shipments, and supporting efficient workflows that reduce consumable waste per result. By enabling laboratories to run detection chlamydia trachomatis testing efficiently, the kit helps minimize instrument idle time and energy use. As labs seek partners who can deliver dependable trachomatis PCR solutions alongside eco-conscious practices, Taizhou Cowingene Biotech Co.,Ltd. stands out as forward-thinking and operationally aligned with modern laboratory and regulatory demands.
Conclusion on detection chlamydia trachomatis,trachomatis pcr,pcr c trachomatis from Taizhou Cowingene Biotech Co.,Ltd.
In today’s molecular diagnostics landscape, reliable detection chlamydia trachomatis solutions are essential for accuracy, throughput, and compliance. Taizhou Cowingene Biotech Co.,Ltd.’s trachomatis PCR kit delivers operational consistency, straightforward workflows, and strong quality controls—supporting labs that prioritize dependable performance and cost efficiency. Backed by rigorous manufacturing and responsive technical support, it’s a smart choice for B2B organizations standardizing pcr c trachomatis testing across sites.
Contact us: email: info@cowingene.com
Visit our website: https://www.cowingene.com
Related PRODUCTS
-
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025 -
Group B Strep PCR Test – Rapid and Accurate Detection for Improved Newborn Health
NewsNov.20,2025







