Oct . 13, 2025 15:35 Back to list
Herpes Simplex Virus Detection | Rapid, Accurate PCR HSV Kit
Field Notes from the Lab: What’s Really Working in herpes simplex virus detection right now
To be honest, the past two years have quietly reshaped HSV testing. Labs want faster qPCR, cleaner specimen workflows, and vendor support that doesn’t ghost you at 5 p.m. One kit I’ve seen popping up in sexual health networks is the Cowingene Herpes simplex virus 1/2 Detection Kit—built for HSV1 and HSV2, simple tube setup, and validated on real-world specimens. It comes out of NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China. Not a household name, but the data and field chatter are interesting.
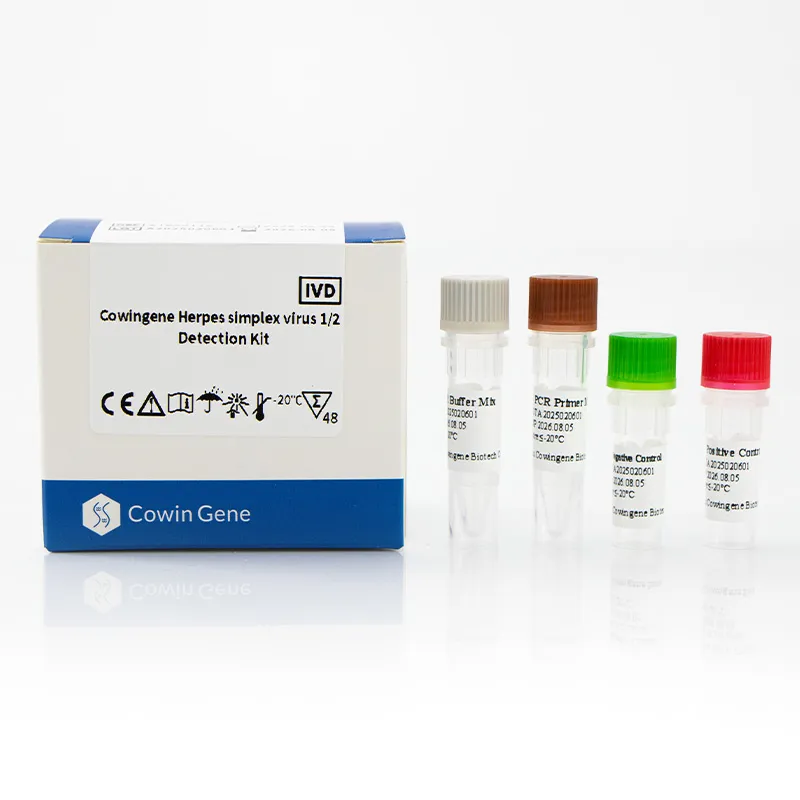
What the kit is (and isn’t)
Cowingene’s REF: ST05011X is a qPCR-based assay that qualitatively detects HSV1/HSV2 DNA in cervical swab, anorectal swab, urine, and self-collected vaginal samples. It’s meant for clinical laboratories—not DIY testing, obviously—and slots into standard extraction + qPCR workflows. In fact, several lab managers told me they liked the single-tube analyte layout; fewer pipetting steps, fewer errors. Still, as with any herpes simplex virus detection product, local validation and QC are non-negotiable.

Technical snapshot
| Product | Cowingene Herpes simplex virus 1/2 Detection Kit |
| REF | ST05011X |
| Targets | HSV1, HSV2 (dual-target, 1 tube) |
| Validated specimens | Cervical swab, Anorectal swab, Urine, Self-collected vaginal |
| Method | Real-time PCR (qPCR) with internal control |
| LoD (internal) | ≈200 copies/mL per target (EP17-A2-style verification; real-world may vary) |
| Run time | ~75–95 min qPCR; total workflow depends on extraction |
| Shelf life / storage | ≈12 months at -20°C (see lot label); avoid >3 freeze–thaw cycles |
| Compliance | Manufactured under ISO 13485; CE/market status subject to region |
Process flow (what labs actually do)
- Materials: validated swabs/urine cups, viral transport medium, extraction kit (magnetic beads OK), qPCR platform.
- Methods: nucleic acid extraction (manual or automated) → master mix prep → one-tube dual-target amplification with internal control.
- Testing standards: verification against CLSI EP17-A2 for LoD; method comparison under CLSI MM03/MM19 guidance; routine IQC/EQC.
- Service life: kits stored -20°C; opened reagents used within recommended on-board time (check IFU).
- Industries: hospital diagnostics, sexual health clinics, public health screens, and research cores.

Why labs pick it (and why some don’t)
Advantages cited: single-tube simplicity, clean curves, and steady supply. One clinic lab reported PPA ≈98.7% and NPA ≈99.2% vs a reference PCR in 312 samples—solid, though sample sets were modest. However, a few users wanted broader specimen claims (e.g., lesion swabs from other sites) and native LIMS files. As always with herpes simplex virus detection, local matrix effects can surprise you.
Quick vendor snapshot (indicative)
| Feature | Cowingene | Vendor A | Vendor B |
| LoD (copies/mL) | ≈200 | ≈300 | ≈150 |
| Time to result | ~90 min qPCR | ~110 min | ~80 min |
| Validated specimens | Cervical, anorectal, urine, self-collected vaginal | Cervical, lesion | Urine, lesion |
| Regulatory | ISO 13485 mfg; region-specific | CE-IVD | CE-IVD |
| Throughput | Batch-friendly, 96-well | Random access | Batch |
| Approx. price/test | ~$ (economy) | $$ | $$$ |
Indicative comparison only; real-world use may vary by lot, instrument, and local validation.
Use cases, customization, and feedback
- Sexual health clinics: same-day herpes simplex virus detection alongside CT/NG panels; lower recollect rates reported.
- Public health pilots: pooled screening (where allowed) to stretch budgets—consult your QA lead first.
- Customization: OEM/white-label, alternative control concentrations, and instrument-specific protocols are available on request.
Customer notes: “Controls behave; no drift across runs.” Another lab said, “Supply chain’s boring—in a good way.” That’s high praise these days.
Compliance and references
Manufacturing under ISO 13485 with traceable lots; typical verification aligned to CLSI guidance. For medical decisions, rely on comprehensive clinical assessment and confirmatory algorithms per local policy—kits like this are one part of the puzzle.
- CDC. Genital Herpes—CDC Fact Sheet. https://www.cdc.gov/std/herpes/stdfact-herpes.htm
- CLSI. EP17-A2: Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures.
- WHO. Essential in vitro diagnostics guidance. https://www.who.int/teams/health-product-policy-and-standards
- ISO 13485:2016 Medical devices—Quality management systems—Requirements for regulatory purposes.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







