Oct . 19, 2025 14:55 Back to list
Influenza A PCR: Fast, Accurate POCT for Rapid Diagnosis
Field Notes on Influenza A PCR: What Labs Are Actually Buying and Why
If you’ve been shopping for influenza a pcr kits this season, you’ve probably noticed two things: supply is better than 2020–22, and expectations are higher. Labs want speed, yes, but also clean data, reliable controls, and sane pricing. I’ve spent weeks comparing options, talking to techs, and running through method sheets. Here’s what stands out about Cowingene’s latest offering—and how it stacks up.
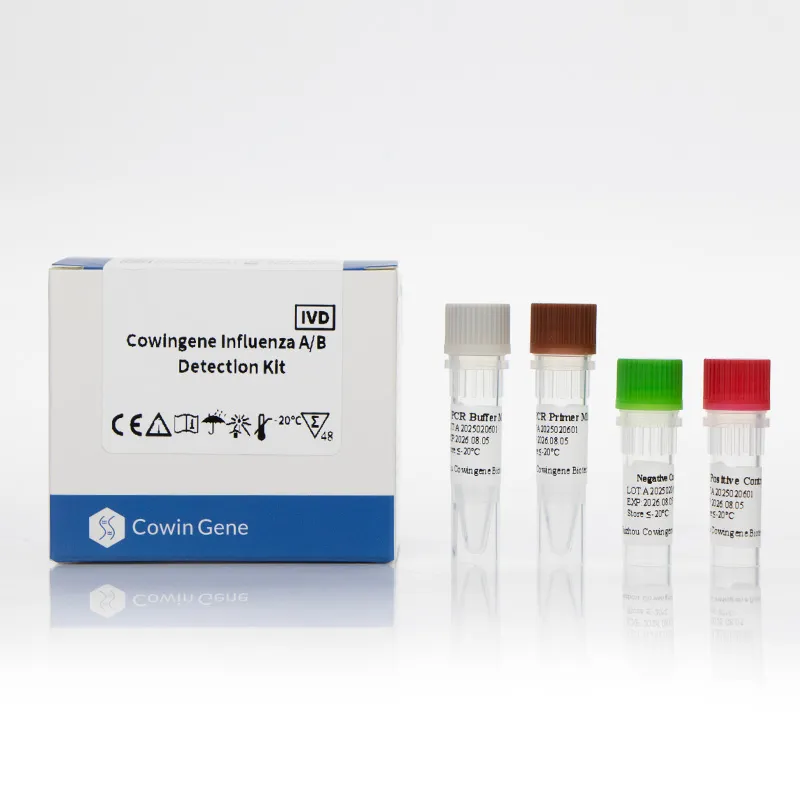
Product snapshot: Cowingene Influenza A/B Detection Kit (Liquid)
From Taizhou, Jiangsu (NO.28, Xinlin Road—yes, I checked), Cowingene’s liquid-format multiplex RT-PCR kit targets Flu A and Flu B in one tube. REF: RP06021X. Validated specimens include nasopharyngeal swab/aspirate, bronchoalveolar lavage, and throat swab. It’s a pragmatic, lab-friendly approach—no flashy gimmicks, just consistent amplification curves. To be honest, that’s what most supervisors tell me they want.
| Name | Cowingene Influenza A/B Detection Kit (Liquid) |
| REF | RP06021X |
| Targets | Influenza A virus (Flu A), Influenza B virus (Flu B) |
| Format | 1-tube multiplex RT‑PCR with internal control |
| Validated specimens | NP swab/aspirate, BAL, throat swab |
| Reaction volume | ≈20–25 µL (instrument dependent) |
| Run time | ≈45–70 min (cycler program) |
| Estimated LoD | Around 200–500 copies/mL; real‑world use may vary (per comparable assays) |
| Storage / Shelf life | -20°C; ≈12 months from manufacture |
| Origin | NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China |
| Compatibility | Common 96‑well qPCR cyclers (e.g., CFX96, 7500 Fast, QuantStudio)† |
| Regulatory | Regional availability; CE/IVD or RUO status varies—confirm locally |
†Check latest instrument list and software versions before validation.
Process flow (what labs actually do)
- Materials: validated swabs/transport media, extraction kit or extraction‑free lysis (lab choice), calibrated qPCR cycler, controls.
- Methods: receive sample → verify ID → extraction/lysis → mix master mix + template → run multiplex RT‑PCR → Ct review.
- QC: include positive/negative and internal controls; track lot trends; verify with CLSI EP05‑A3 (precision) and EP17‑A2 (LoD).
- Acceptance: control Ct within ranges; no amplification in NTC; replicate agreement within lab SOP.
- Service life: reagents stable until expiry; avoid freeze‑thaw cycles (>3 is pushing it).
- Industries: hospitals, public health labs, urgent care networks, airlines/ports (screening), pharma studies (exploratory).
How it compares (quick vendor snapshot)
| Vendor / Assay | Method | Time | Throughput | Best for | Notes |
|---|---|---|---|---|---|
| Cowingene Flu A/B (Liquid) | Multiplex RT‑PCR | ≈45–70 min | Batch, 96‑well | Core labs balancing cost and scale | Flexible instruments; competitive pricing |
| Roche cobas Liat Flu A/B | RT‑PCR cartridge | ≈20 min | POC, single‑sample | ED/urgent care | Fast; higher per‑test cost |
| Abbott ID NOW Flu | Isothermal NAAT | ≈13 min | POC | Clinics needing speed | Convenient; sensitivity can vary |
| Cepheid Xpert Xpress Flu/RSV | RT‑PCR cartridge | ≈30 min | Moderate (GeneXpert) | Hospitals with GeneXpert | Reliable; instrument‑locked |
Applications and real‑world feedback
- Hospital labs: batch up to 94 patient samples plus controls during peak hours—many customers say the internal control behavior is “predictable,” which is rarer than it should be.
- Public health: during cluster investigations, multiplexing Flu A/B in one run saves staff time. I guess that’s why it’s showing up in county bids.
- Case mini‑study 1: A regional lab cut turnaround from 24h to same‑day by moving to influenza a pcr batch runs in the morning and late afternoon.
- Case mini‑study 2: An employer clinic partnered with a reference lab; influenza a pcr confirmed antigen‑screen positives, reducing false alarms and needless sick leave.
Standards, validation, and data expectations
Most labs validate against CLSI EP17‑A2 (LoD), EP05‑A3 (precision), and MM03/MM19 for nucleic acid methods, with CDC Influenza RT‑PCR protocol elements as anchors. Expect clean negatives (NTC), reproducible Ct shifts (influenza a pcr demand spikes in Q4–Q1 every year; stock accordingly.
Bottom line
If you need flexible, batchable influenza a pcr without being locked into a cartridge ecosystem, Cowingene’s kit is a solid, no‑drama option. It’s not the flashiest, but it’s practical—and that’s usually what keeps labs on schedule.
Citations
- CDC. CDC Real‑Time RT‑PCR (rRT‑PCR) Protocol for Influenza A/B. https://www.cdc.gov/flu
- WHO. Molecular detection of influenza viruses. https://www.who.int/influenza
- CLSI. EP17‑A2: Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures.
- CLSI. EP05‑A3: Evaluation of Precision of Quantitative Measurement Procedures.
- ISO 13485:2016 Medical devices—Quality management systems—Requirements for regulatory purposes.
Related PRODUCTS
-
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025 -
Group B Strep PCR Test – Rapid and Accurate Detection for Improved Newborn Health
NewsNov.20,2025







