Sep . 30, 2025 15:30 Back to list
Looking for MTB Detection with a Rapid, Sensitive PCR Kit?
Real‑world MTB detection for busy labs: notes from the field
If you’ve been following tuberculosis diagnostics, you know the market’s shifting fast—toward faster, simpler workflows that still pass audit. For anyone benchmarking mtb detection this season, here’s a grounded look at a liquid-format kit that’s been getting attention: the Cowingene MTB and NTM Detection Kit (Liquid). To be honest, what caught my eye first was the one‑tube analyte design; many customers say it lowers hands-on steps without dumbing things down.
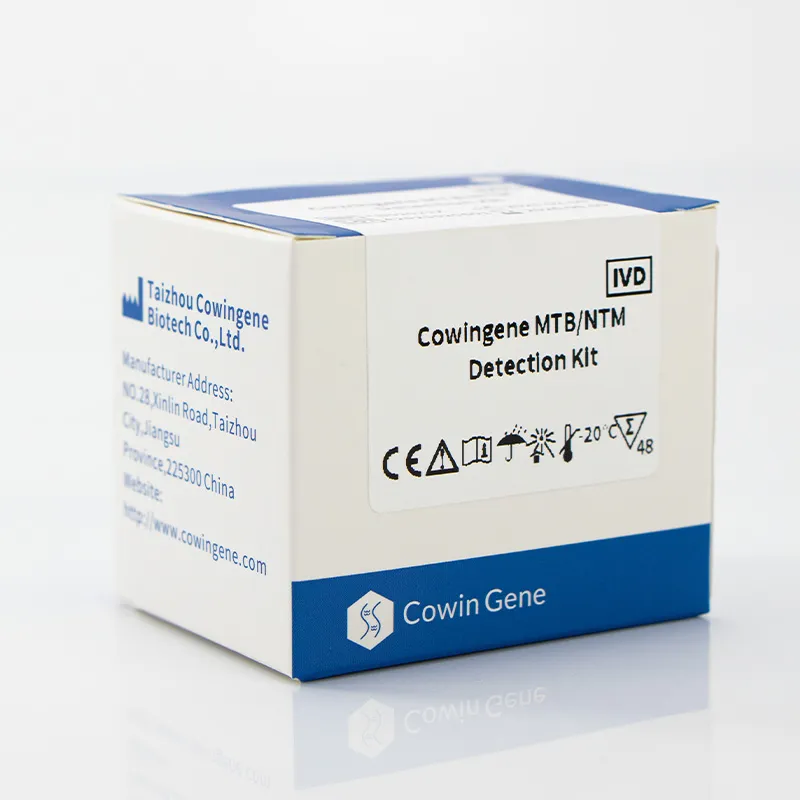
Product snapshot and specs
From Jiangsu Province, China (NO.28, Xinlin Road, Taizhou City), the kit targets Mycobacterium tuberculosis (MTB) alongside nontuberculous mycobacteria (NTM). It’s positioned for sputum, bronchial washings, and cultured cell specimens—so the usual respiratory suspects.
| Product name | Cowingene MTB and NTM Detection Kit (Liquid) |
| REF | TB02021X |
| Validated specimens | Sputum, Bronchial washing, Cultured cell |
| Analytes / format | 1 tube; MTB and NTM |
| Methodology | PCR-based detection (qPCR-style workflows are commonly used; confirm in IFU) |
| Shelf life | Per lot COA; typical industry range ≈12–18 months (storage conditions may vary) |
| Compliance | Manufacturers usually align to ISO 13485 and labs to ISO 15189; verify certificates |
Where it fits: application scenarios
- Hospital microbiology labs needing same‑day mtb detection alongside NTM screening.
- Public health and TB reference centers scaling seasonal surges.
- Occupational screening programs and outbreak investigations (with lab confirmation).
Process flow (materials, methods, QC)
A typical lab workflow I see in the wild looks like this (your IFU rules, of course):
- Specimen prep: decontamination and concentration (e.g., NALC‑NaOH); include an extraction control.
- Nucleic acid extraction: magnetic beads or column kits; elute ~50–100 µL.
- Amplification: load master mix + template; run qPCR thermal profile per kit IFU.
- Controls and standards: positive/negative controls; verify Ct cutoffs; document runs.
- Interpretation: MTB vs NTM channels; inconclusive repeats per CLSI MM03 guidance.
Testing standards to anchor your SOPs: WHO TB diagnostics guidance for rapid tests, CLSI MM03 for molecular infectious disease methods, and ISO 15189 for lab competence. Service life and storage? Check the COA; real‑world stability can vary with cold‑chain handling.
What labs report (advantages and a pinch of data)
- One‑tube workflow lowers pipetting errors, it seems, especially with new staff.
- Turnaround often lands within a single shift; some sites quote 2–4 hours end‑to‑end (extraction included).
- Typical qPCR LODs in literature for MTB range roughly 10–100 CFU/mL; real‑world use may vary by matrix and extraction.
Vendor landscape (quick comparison)
| Vendor / Kit | Targets | Format | TAT (≈) | Notes |
|---|---|---|---|---|
| Cowingene MTB & NTM (Liquid) | MTB + NTM | PCR kit, 1‑tube analyte | 2–4 h (lab‑dependent) | Open platform flexibility; confirm instrument list |
| Cepheid Xpert MTB/RIF Ultra | MTB + RIF resistance | Cartridge, closed system | ~1.5–2 h | Low training burden; instrument‑locked |
| Roche cobas MTB | MTB | Automated, high‑throughput | Batch‑dependent | Great for central labs; higher capital outlay |
Customization and support
OEM/white‑label, language‑specific IFUs, and kit sizes are commonly offered in this category. If you’re harmonizing multiple labs, ask about cross‑lot bridging studies, panel compatibility, and whether mtb detection and NTM channels can be tuned to your reporting thresholds.
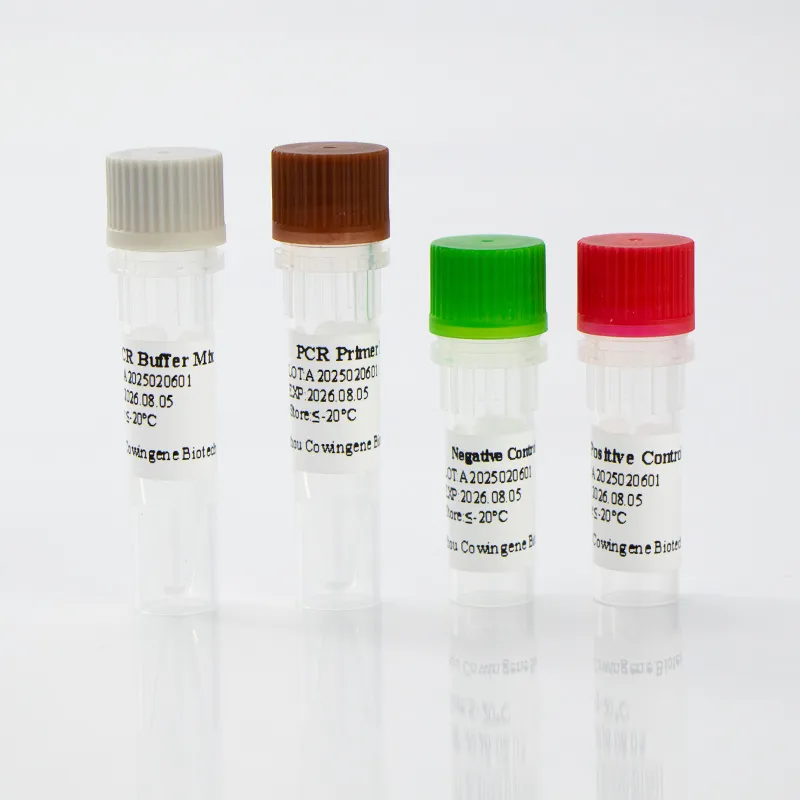
Field note (mini case)
A mid‑sized respiratory lab I visited swapped an aging assay for a liquid kit with a one‑tube setup. Their internal audit (three months in) showed a 30% reduction in repeats and smoother onboarding for new techs. Not a randomized trial—just practical evidence that simpler mtb detection workflows can pay off.
Standards and citations
- World Health Organization. Consolidated guidelines on TB. Module 3: Diagnosis – Rapid diagnostics. 2021/2024 updates. https://www.who.int/publications
- CLSI. MM03—Molecular Diagnostic Methods for Infectious Diseases (latest ed.). https://clsi.org
- ISO 15189:2022 Medical laboratories — Requirements for quality and competence. https://www.iso.org
- ATS/ERS/ESCMID/IDSA Clinical Practice Guideline for Nontuberculous Mycobacterial Pulmonary Disease, 2020. https://www.idsociety.org
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







