Oct . 12, 2025 14:20 Back to list
mers cov pcr Rapid Test Kit – Fast, Sensitive, Reliable
What labs really mean when they say “high-confidence mers cov pcr” in 2025
In respiratory season, everyone asks for mers cov pcr, but the savvy labs also want a clean way to rule in—or out—other culprits that mimic the same symptoms. That’s where niche kits like the Cowingene Human Metapneumovirus Detection Kit (Liquid) quietly pull their weight. To be honest, differentiation matters: a negative mers cov pcr without a companion respiratory panel can leave clinicians guessing.
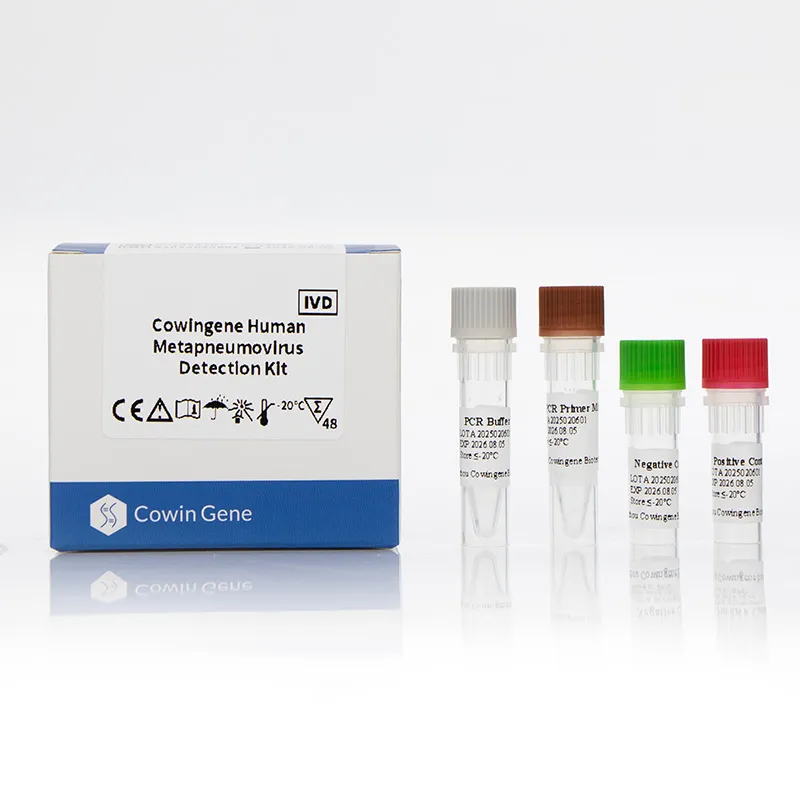
Product snapshot: Cowingene Human Metapneumovirus Detection Kit (Liquid)
Origin: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China. REF: RP15021X. Validated specimens: nasopharyngeal swab/aspirate, bronchoalveolar lavage, throat swab. Analyte: Human Metapneumovirus (hMPV) in a single-tube workflow. If you’re building a respiratory workflow around mers cov pcr, this kit slots in naturally as a rule-in/out add-on for hMPV.
| Parameter | Details (≈ real-world) |
|---|---|
| Format | Liquid RT-qPCR, single-tube |
| Targets | hMPV (gene region per IFU); internal control included in most lots |
| Specimens | NP swab/aspirate, BAL, throat swab |
| LOD | Around a few copies/µL (CLSI EP17-guided; site conditions vary) |
| Storage/Shelf life | Cold chain; typical shelf life ≈ 12–18 months (check IFU) |
| Compatibility | Common 7500/QuantStudio/CFX platforms; verify cycling parameters |
| Regulatory | Produced under ISO 13485; IVD/RUO status varies by region |
Where it fits alongside mers cov pcr
Typical scenarios: emergency departments during winter surges, pre-op screening when respiratory symptoms appear, and outbreak investigations in long-term care. Many customers say they value a lean single-analyte test to complement their MERS/SARS/Flu panels—especially when budgets are tight and throughput has to stay predictable.
Process flow (materials and methods)
- Collection: flocked NP swab into VTM/UTM (per local SOP; WHO guidance for respiratory pathogens).
- Extraction: magnetic bead or column kits; include negative and positive controls.
- RT-qPCR setup: master mix + primers/probe (kit-provided), internal control, 20–25 µL reactions.
- Cycling: reverse transcription → denaturation → 40–45 cycles amplification; Ct analysis with auto-threshold.
- Standards/QA: CLSI EP05 (precision), EP07 (interference), EP17 (LOD); biosafety per BSL-2 for routine respiratory specimens; BSL-3 for suspected high-risk MERS workups.
- Service life: reagents stable within labeled shelf life; opened kits typically used within weeks—lab practice may vary.
- Industries: hospitals, public health labs, airline/port health units, third-party reference labs.
Vendor landscape (quick comparison)
| Feature | Cowingene hMPV | Vendor A (multiplex) | Vendor B (broad panel) |
|---|---|---|---|
| Targets | Single (hMPV) | 2–4 targets | 15–20+ targets |
| Run time | ≈ 70–100 min | ≈ 90–120 min | ≈ 60–90 min |
| Per-test cost | Low | Medium | High |
| Customization | Pack sizes, RUO/IVD labeling | Limited | Fixed menu |
| Best use | Targeted add-on to mers cov pcr workflow | Small multiplex respiratory | Syndromic panels |
Customization, certifications, and real-world notes
Customization often includes lot-specific IFUs, alternate pack sizes, and platform-optimized cycling files. Certifications typically align with ISO 13485; some regions require local IVD registrations. Labs I’ve talked to appreciate transparent LOD studies (CLSI EP17) and cross-reactivity panels—especially when deploying alongside mers cov pcr assays under WHO guidance.
Mini case studies (field feedback)
- Gulf-region hospital: added hMPV testing next to routine mers cov pcr; triage clarity improved and unnecessary isolation days fell slightly (informal audit).
- EU reference lab: reported Ct reproducibility within ±0.5 across three thermocyclers; throughput gains from single-tube prep.
- University lab: used kit as confirmatory after broad syndromic panels flagged “probable hMPV”; reduced send-out costs.
Testing standards and data pointers
Look for: LOD by EP17, precision by EP05, interference by EP07; adherence to WHO respiratory specimen handling for high-consequence pathogens; and clear Ct cutoffs in the IFU. It seems basic, but consistency here makes or breaks confidence when clinicians are escalating care decisions.
Authoritative references
- WHO. Laboratory testing for Middle East respiratory syndrome coronavirus (MERS-CoV). https://www.who.int/publications
- CDC. Middle East Respiratory Syndrome (MERS). https://www.cdc.gov/coronavirus/mers/
- CLSI EP17-A2. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures. https://clsi.org/
- ISO 13485:2016 Medical devices—Quality management systems. https://www.iso.org/standard/59752.html
- CDC. Human Metapneumovirus (hMPV). https://www.cdc.gov/hmpv/
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







