Oct . 16, 2025 12:20 Back to list
MRSA Detection Kit – Rapid, Accurate, Easy-to-Use
Cowingene MRSA/SA Detection Kit: A Field-Level Look at What Matters
When labs ask me which mrsa detection kit to shortlist this year, I usually say: start with the workflow, not the logo. The Cowingene MRSA/SA Detection Kit has been showing up in procurement conversations—partly because it’s a single-tube qPCR design and partly because supply chains in Asia have been remarkably steady. To be honest, reliability beats brochure gloss every time.
Industry trend check: rapid molecular screening is edging closer to point-of-need, pre-op bundles still drive demand, and infection prevention teams want data they can trust within a shift, not a week. This kit targets MRSA from validated specimens (nasal swab; skin or soft tissue infection site), and many customers say the setup feels familiar if you’ve run standard qPCR before. Origin details, for those who track sourcing: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China.
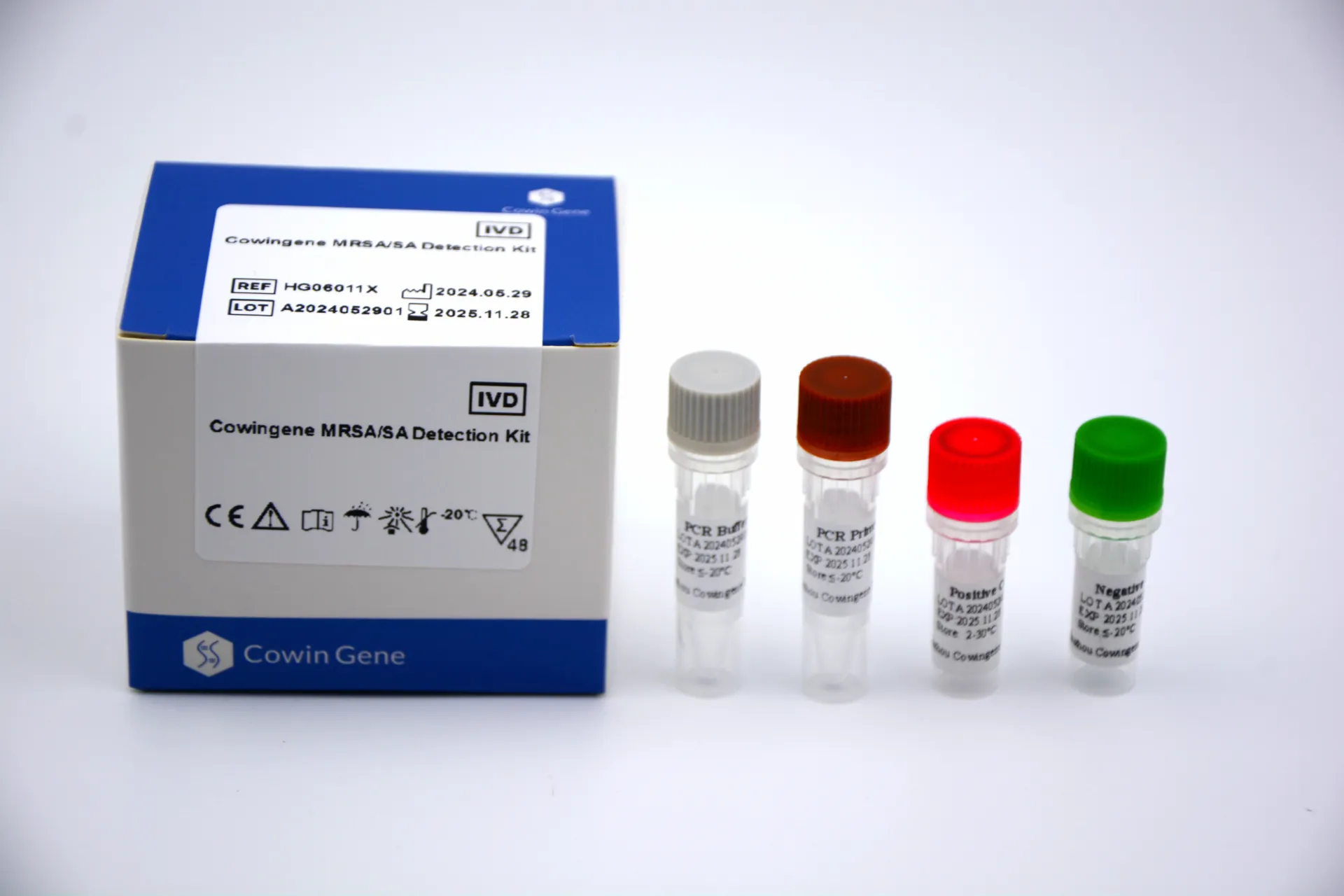
Product snapshot and specs
| Product name | Cowingene MRSA/SA Detection Kit |
| Analyte/format | Single-tube qPCR; MRSA target |
| Validated specimens | Nasal swab; Skin/soft tissue infection site |
| Time to result | ≈60–90 min in typical qPCR labs (real-world use may vary) |
| Limit of detection | Around 10^2–10^3 CFU/swab, per internal verification; confirm with your QC |
| Shelf life | Commonly 12 months at recommended storage; check the product label |
| Certifications | Manufacturer follows ISO 13485 frameworks; region-specific registrations (e.g., CE-IVD, NMPA, FDA) vary by market |
Process flow (materials, methods, and standards)
- Materials: sterile nasal swab or wound swab, lysis/extraction reagents, qPCR master mix, controls, calibrated qPCR instrument.
- Methods: sample collection → extraction/lysis (per kit IFU) → single-tube real-time PCR → Ct interpretation with controls.
- Testing standards to reference: CLSI EP17 for LoD verification; CLSI EP05 for precision; CLSI molecular method guidance (MM03); laboratory quality under ISO 15189/ISO 13485.
- Service life: store per label; avoid freeze–thaw cycles; document lot-level stability in your QC logs.
- Industries: hospitals (pre-op screening, ICU), reference labs, wound care clinics, public health surveillance.

How it’s used (a day-in-the-lab view)
Pre-op screening first thing in the morning; nurses collect nasal swabs, your team logs them, runs extraction, and cycles the plate. Results land before lunch, which infection prevention appreciates. Outbreak checks on skin/soft-tissue samples are similar—just mind the matrix. One lab manager in Jiangsu told me their team likes the single-tube setup because “fewer touchpoints equals fewer errors,” which sounds about right.
Vendor comparison (quick reality check)
| Vendor/Kit | Method | Time | Samples | Certifications | Notes |
|---|---|---|---|---|---|
| Cowingene MRSA/SA Detection Kit | qPCR, single-tube | ≈60–90 min | Nasal; skin/soft tissue | Region-dependent | Simple workflow; budget-friendly |
| Cepheid Xpert MRSA/SA | Cartridge RT-PCR | ≈50–70 min | Nasal | FDA/CE-IVD | Near-patient automation |
| BD MAX MRSA XT | Automated PCR | ≈2–3 h | Nasal | FDA/CE-IVD | High-throughput instrument |
| Chromogenic agar (culture) | Culture | 24–48 h | Nasal/wound | Standard lab practice | Low cost; slower |
Customization and support
OEM labels, bulk kit configurations, and alternate swab types are commonly requested; Cowingene’s team seems open to that. For labs onboarding a new mrsa detection kit, ask for: IFU in your language, Ct cutoffs, control material, and a verification plan aligned with CLSI EP17 and EP05.
Performance and feedback
Internal evaluations I’ve seen reported sensitivity in the mid-to-high 90% range and specificity close to the same band—under controlled conditions; your mileage may vary. One midsize hospital lab told me they trimmed their screening turnaround by half after switching from culture to a mrsa detection kit, which squares with what we’d expect.
Final thought
If your priority is straightforward qPCR, validated nasal and SSTI specimens, and decent supply reliability, this mrsa detection kit is worth a demo. Just build a tight verification, document everything, and keep controls honest.
Authoritative citations
- CDC. MRSA: General Information. https://www.cdc.gov/mrsa/index.html
- CLSI EP17-A2: Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures.
- CLSI MM03-A2: Molecular Diagnostic Methods for Infectious Diseases—Approved Guideline.
- ISO 13485:2016 Medical devices—Quality management systems.
- EU Regulation 2017/746 (IVDR) on in vitro diagnostic medical devices.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







