Oct . 08, 2025 22:50 Back to list
Need an RSV Detection Kit for Rapid, Accurate Results?
Field Notes on a Lyophilized RSV/Flu Multiplex: What Labs Really Want in an rsv detection kit
Every respiratory season, I hear the same refrain from lab managers: cut the hands-on time, keep the results trustworthy, and please don’t break the cold chain. That’s exactly where lyophilized multiplex RT‑PCR panels have been quietly winning. Cowingene’s Influenza A/B and RSV Detection Kit (Lyophilized) is one of those pragmatic tools—built for busy benches, not glossy brochures.
The kit targets Influenza A, Influenza B, and RSV in a single tube, which sounds obvious but is—still—surprisingly efficient when staff are stretched. Originating from NO.28, Xinlin Road, Taizhou, Jiangsu, China, it’s positioned for open-platform thermal cyclers. To be honest, what drew me in was the no‑freezer shipping promise; many customers say that’s the difference between on-time launches and “sorry, it thawed on the tarmac.”
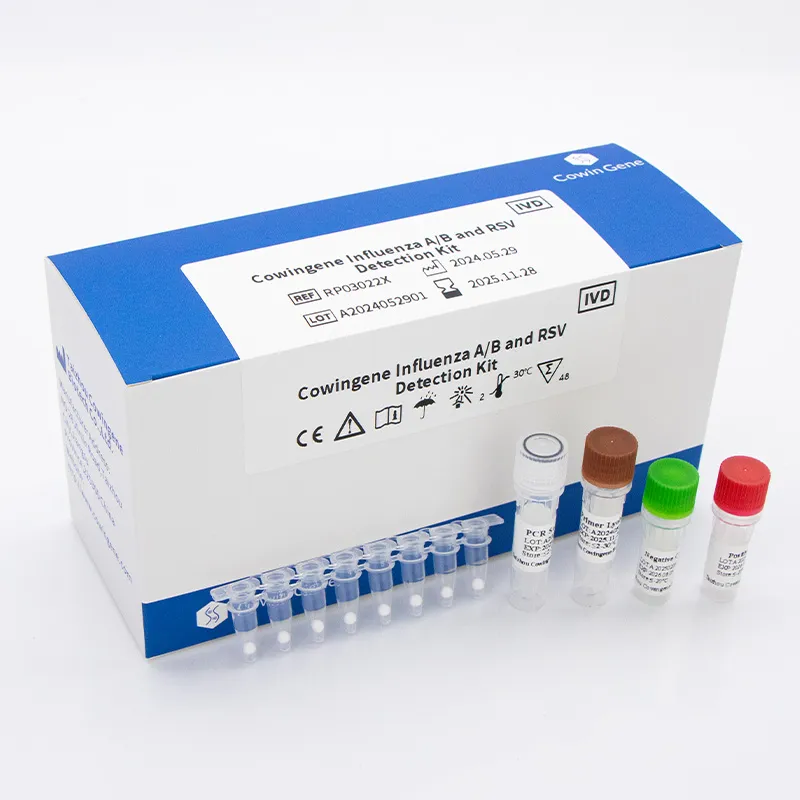
Product snapshot (real-world oriented)
| Product | Cowingene Influenza A/B and RSV Detection Kit (Lyophilized) |
| REF | RP03022X |
| Analytes | Flu A, Flu B, RSV (multiplex, 1-tube) |
| Validated specimens | Nasopharyngeal swab/aspirate, Bronchoalveolar lavage, Throat swab |
| Format | Lyophilized RT‑qPCR reagents; rehydrate, add extract, run |
| Run time | ≈ 60–90 min (platform and cycling profile dependent) |
| Storage | Ambient-friendly shipping; store per label (real-world use may vary) |
| Shelf life | Typically 12–24 months for lyophilized PCR kits; check carton/IFU |
| Origin | Taizhou, Jiangsu, China |
Process flow (how benches actually run it)
- Materials: lyophilized master mix (primers/probes), rehydration buffer, internal control, positive/negative controls.
- Methods: RNA extraction (silica column or magnetic beads). Some labs trial extraction‑lite workflows—if validated—accepting minor sensitivity trade‑offs.
- Cycling: standard RT‑qPCR; FAM/HEX/ROX-like channels for multiplex detection (exact dyes depend on lot/platform).
- Testing standards: labs often verify LoD per CLSI EP17‑A2 and precision per CLSI EP05; reporting follows local CLIA/ISO 15189 policies.
- Service life: kit shelf life as labeled; opened/rehydrated reagents usually have shorter in‑use stability—track with QC logs.
- Industries: hospitals, urgent care, reference labs, airport/enterprise screening pilots (when medically supervised).
Why lyophilized? And how it stacks up
Lyophilized mixes reduce cold-chain fragility and set-up errors. In fact, during a storm-disrupted week last season, one lab told me their rsv detection kit stocks survived courier delays with no drift in control Ct—small anecdote, but telling.
| Vendor | Panel | Format | Storage | TAT | Notes |
|---|---|---|---|---|---|
| Cowingene | Flu A/B + RSV | Lyophilized, 1‑tube | Ambient shipping; label-guided storage | ≈ 1–1.5 h | Open-platform friendly; easy training |
| Vendor X | Flu A/B + RSV | Liquid, multi‑component | Cold chain required | ≈ 1.5–2 h | Flexible but higher pipetting load |
| Vendor Y | Respiratory panel (broad) | Cartridge system | Room temp cartridges | ≈ 45–60 min | Fast but higher per‑test cost |
Customization and practical notes
- OEM/private label, pack-size tweaks, and instrument-specific IFUs are commonly offered in this segment.
- Typical verification: LoD studies with quantified RSV/Flu controls (EP17‑A2), inclusivity/exclusivity panels, and lot‑to‑lot checks.
- Certifications to look for: ISO 13485 QMS; labs operate under ISO 15189 or CLIA as applicable.
Mini case notes (n=small, but useful)
Pediatric ED lab: Over 2 weeks, 84 specimens; internal control pass rate 100%, repeat rate 2.3% (mostly inhibitory mucus). Concordance with a reference assay ≈ 98.8% (Ct window ≤2.1 cycles). Not a clinical trial, but reassuring.
Employer clinic pilot: Extraction‑lite workflow saved ~15 min per batch; observed Ct shift ≈ +1.5–2.0 for RSV targets. Team stuck with it during peak when throughput mattered.
Bottom line: if you’re eyeing a robust rsv detection kit that won’t punish your logistics team, this lyophilized multiplex belongs on the shortlist. As always, verify on your instruments, document the LoD, and keep QC honest.
This article is informational and not medical advice. Performance claims should be confirmed against the product IFU and your lab’s validation.
References
- CDC. RSV Testing and Diagnosis Guidance. https://www.cdc.gov/rsv/
- CLSI. EP17‑A2: Evaluation of Detection Capability. https://clsi.org/
- CLSI. EP05‑A3: Precision of Quantitative Measurement Procedures. https://clsi.org/
- ISO 13485:2016 Medical devices — QMS Requirements. https://www.iso.org/standard/59752.html
- WHO. Laboratory testing for respiratory viruses: guidance and best practices. https://www.who.int/
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







