Oct . 22, 2025 17:30 Back to list
Respiratory Panel Test for Rapid, Accurate PCR Results
Inside the Lab: A closer look at Cowingene’s lyophilized SARS‑CoV‑2 & Influenza A/B panel
If you’ve been hunting for a [Respiratory Panel Test For] high‑throughput clinical screening, you’ve probably noticed the market shifting. Multiplex RT‑qPCR is now the default in many labs, and lyophilized chemistry is quietly solving the cold‑chain headache. To be honest, that last bit matters more than most people think—especially for regional labs and field deployments.
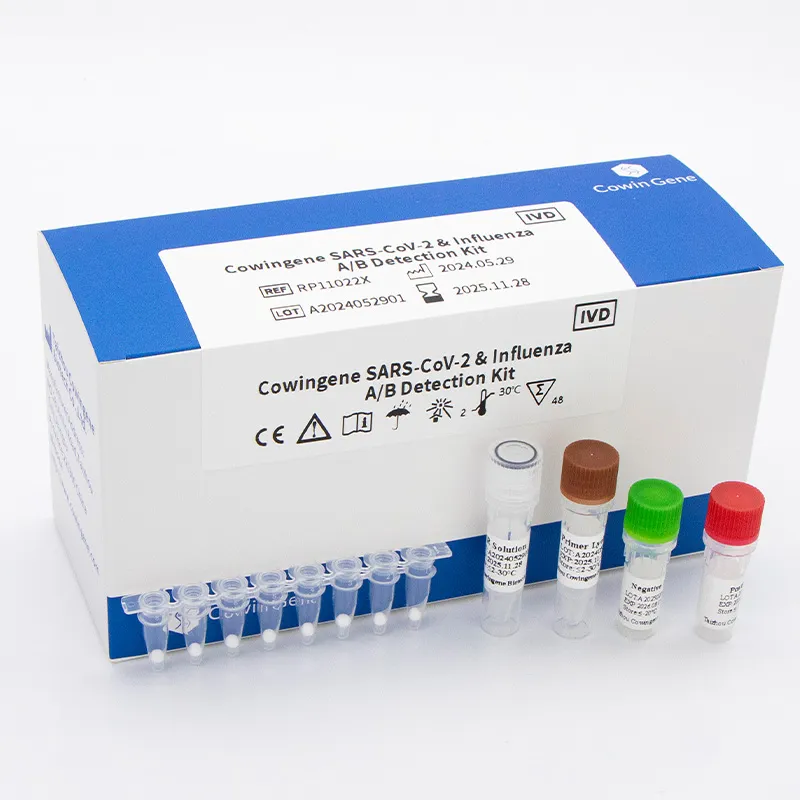
Cowingene’s SARS‑CoV‑2 & Influenza A/B Detection Kit (Lyophilized), REF: RP11022X, targets three analytes—SARS‑CoV‑2, Flu A, and Flu B—across common respiratory specimens (nasopharyngeal swab/aspirate, throat swab, bronchoalveolar lavage). It’s built for routine hospital workflows, but I’ve also seen it used in pop‑up winter clinics where staff rotate and consistency counts. Many customers say the “open, add water, run” feel of lyophilized beads reduces pipetting stress. I guess that’s the charm: fewer freeze‑thaw cycles, cleaner benches.
Product specifications (snapshot)
| Product | Cowingene SARS‑CoV‑2 & Influenza A/B Detection Kit (Lyophilized) |
| REF | RP11022X |
| Method | Multiplex real‑time RT‑PCR (lyophilized reagents; rehydrate then run) |
| Analytes | SARS‑CoV‑2, Influenza A, Influenza B |
| Validated specimens | Nasopharyngeal swab/aspirate, Bronchoalveolar lavage, Throat swab |
| Throughput & TAT | 96‑well instruments; ≈80–120 min runtime (real‑world use may vary) |
| Storage & shelf life | Lyophilized format; follow IFU. Typical shelf life ≈12 months (verify lot‑specific data). |
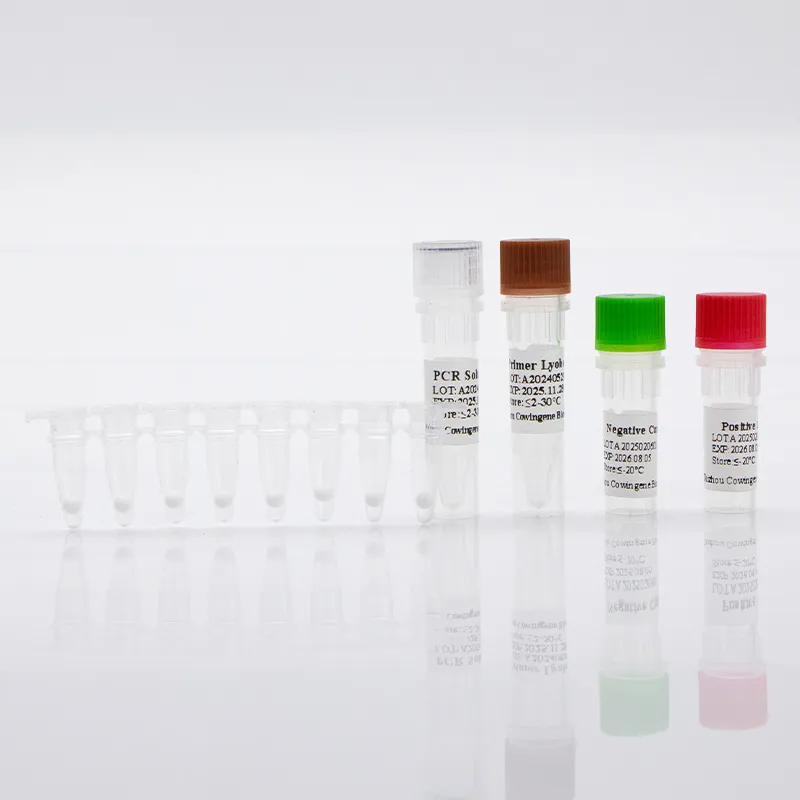
| Controls | Internal control; external positive/negative as per IFU |
| Origin | NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China |
Process flow (how labs actually run it)
- Collect specimen with approved swabs; transport in VTM/UTM per lab SOP.
- Extraction: magnetic beads or column kits; some sites validate extraction‑free for swabs (confirm locally).
- Rehydrate lyophilized mix, add template, seal plate/tubes.
- RT‑qPCR cycling on a validated thermocycler; analyze Ct vs. channel calls per IFU.
- QC against internal control; batch acceptance using CLSI EP05/EP15 practices.
Standards worth mapping to: MIQE for qPCR reporting, CLSI EP17 for LoD, and your lab’s ISO 15189 framework. For conformity and import, request ISO 13485 manufacturing documentation and any regional listings. Regulatory status varies by country—always verify.
Where it fits (and why lyophilized matters)
Hospitals juggling influenza‑like illness, university health centers during peak season, and provincial labs short on freezer space—these are classic scenarios. Multiplexing reduces resampling, which patients appreciate. And yes, [Respiratory Panel Test For] busy triage lines is a small win in winter.
Vendor comparison (practical view)
| Criteria | Cowingene (RP11022X) | Brand T (global) | Brand S (global) |
|---|---|---|---|
| Analytes | SARS‑CoV‑2, Flu A/B | SARS‑CoV‑2, Flu A/B | SARS‑CoV‑2, Flu A/B |
| Format | Lyophilized | Liquid (often) | Lyophilized or liquid |
| Cold chain | Reduced burden | Higher burden | Mixed |
| TAT (PCR) | ≈80–120 min | ≈70–120 min | ≈80–120 min |
| Customization/OEM | Available on request | Limited | Project‑based |
Customization and real‑world notes
OEM branding, kit sizes, and instrument‑specific validation packages can usually be arranged. Some buyers ask about adding RSV; that’s typically a separate panel, but bundling in the supply contract is common. For a [Respiratory Panel Test For] tender, request: IFU, LoD study (per CLSI EP17), cross‑reactivity/interference data, and instrument compatibility list.
Mini case study
A coastal community hospital ran this kit over a winter surge on a standard 96‑well cycler. Lab leads reported fewer repeats after switching from single‑pathogen assays and flagged several coinfections that altered isolation decisions. Feedback was surprisingly down‑to‑earth: “Less fiddly pipetting” and “stock survives courier delays.” Not flashy, but that’s what keeps lines moving.
[Respiratory Panel Test For] procurement checklist: confirm regulatory status for your market, align QC with MIQE and ISO 15189, verify LoD and inclusivity/exclusivity, and—this is key—stress‑test logistics with lyophilized lots across your shipping lanes.
References
- CLSI. EP17‑A2: Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures.
- Bustin SA et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real‑Time PCR Experiments. Clin Chem. 2009.
- WHO. Diagnostic testing for SARS‑CoV‑2: interim guidance (latest revision).
- CDC. Influenza SARS‑CoV‑2 (Flu SC2) Multiplex Assay Resources (EUA documentation).
- ISO 15189:2022 Medical laboratories — Requirements for quality and competence.
Related PRODUCTS
-
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025 -
Group B Strep PCR Test – Rapid and Accurate Detection for Improved Newborn Health
NewsNov.20,2025







