Oct . 06, 2025 04:15 Back to list
Respiratory syncytial virus by PCR: fast, accurate testing?
respiratory syncytial virus by pcr,respiratory syncytial virus rna pcr,rsv pcr positive is a key solution in the healthcare industry, specifically within medical device and in vitro diagnosis. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
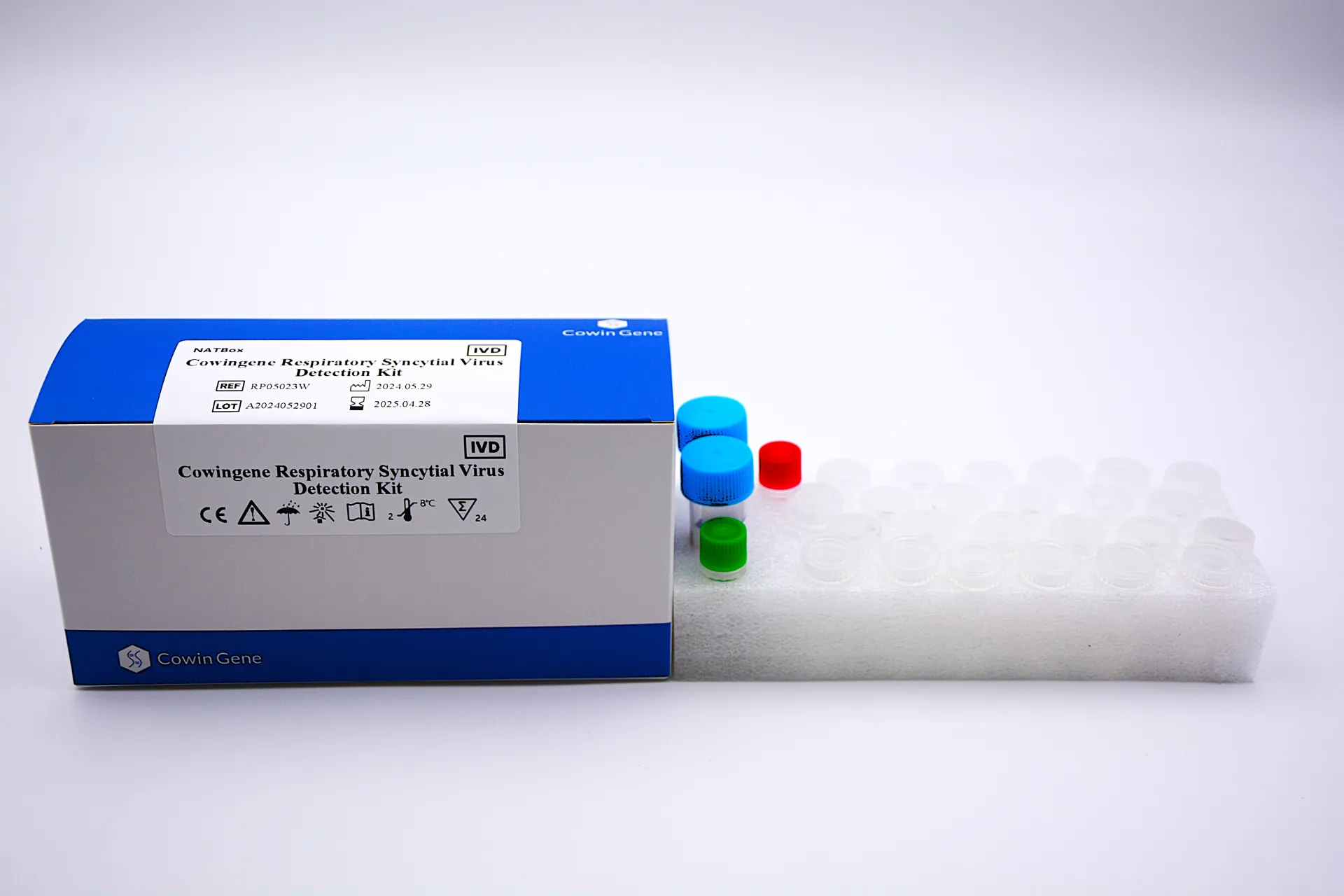
Table of Contents
- respiratory syncytial virus by pcr,respiratory syncytial virus rna pcr,rsv pcr positive Overview
- Benefits & Use Cases of respiratory syncytial virus by pcr,respiratory syncytial virus rna pcr,rsv pcr positive in in vitro diagnosis
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in healthcare
- Conclusion on respiratory syncytial virus by pcr,respiratory syncytial virus rna pcr,rsv pcr positive from Taizhou Cowingene Biotech Co.,Ltd.
respiratory syncytial virus by pcr,respiratory syncytial virus rna pcr,rsv pcr positive Overview
Respiratory syncytial virus by PCR refers to the qualitative detection of RSV RNA using real-time reverse transcription polymerase chain reaction (RT‑PCR). In an in vitro diagnosis setting, respiratory syncytial virus RNA PCR targets conserved genomic regions (commonly N or F genes) and measures fluorescence signals to determine the presence of viral RNA in upper respiratory specimens such as nasopharyngeal swabs. This method is valued for analytical sensitivity, specificity, and rapid turnaround—typically achievable within a single laboratory shift—making it useful across pediatric and adult care pathways.
Taizhou Cowingene Biotech Co.,Ltd. provides a robust RSV RT‑PCR assay designed for streamlined workflows on standard real-time PCR instruments. The kit architecture typically includes an integrated internal control to monitor extraction and amplification efficiency, helping laboratories reduce invalid runs. Closed‑tube detection minimizes contamination risk, while clear result interpretation guides when a sample is RSV PCR positive or negative. Backed by disciplined manufacturing and quality processes, Cowingene aligns its product design to the expectations of hospital, reference, and public health laboratories that require reliable, repeatable results.
Benefits & Use Cases of respiratory syncytial virus by pcr,respiratory syncytial virus rna pcr,rsv pcr positive in in vitro diagnosis
For clinical laboratories, respiratory syncytial virus RNA PCR supports timely decisions during seasonal surges and year‑round surveillance. Use cases span emergency departments, pediatric wards, and outpatient networks where accurate differentiation from other respiratory pathogens informs infection control and cohorting. When results are RSV PCR positive, clinicians can prioritize appropriate patient management and resource allocation, while negative results can prompt reflex testing for other etiologies or multiplex panels.
Taizhou Cowingene Biotech Co.,Ltd.’s solution emphasizes operational continuity: compatibility with common extraction chemistries and thermocyclers, intuitive result readouts, and internal control monitoring. Laboratories benefit from streamlined reagent handling and lot‑to‑lot consistency, which reduces repeat testing and variability. Compared with antigen methods, respiratory syncytial virus by PCR supports earlier detection in the infection timeline and offers high analytical performance, particularly when sample quality and pre‑analytical steps are well controlled. In B2B contexts—IDNs, lab networks, and diagnostics distributors—these advantages translate into scalable testing capacity and dependable reporting across diverse sites.
Cost, Maintenance & User Experience
Total cost of ownership for RSV RT‑PCR hinges on three levers: reagent efficiency, instrument utilization, and staff time. Cowingene’s kit is built for low-hassle setup and predictable throughput, helping labs run more samples per shift without increasing labor. Standardized components and clear protocols can reduce training time and mitigate errors, while internal controls help avoid costly re‑runs. When workflows are optimized, laboratories often see lower cost per reportable result and improved turnaround times.
From a user experience perspective, customers in the medical device sector value consistency and minimal maintenance. The assay’s closed‑tube RT‑PCR format limits contamination risk and supports stable performance over many cycles of use. Feedback from professional users typically emphasizes reliable amplification curves, straightforward interpretation of RSV PCR positive results, and compatibility with routine quality processes. For B2B decision makers, the ROI emerges through fewer invalids, better instrument uptime, consolidated procurement, and the ability to flex capacity during RSV season without compromising quality.
Sustainability & Market Trends in healthcare
Global respiratory testing is evolving toward smarter capacity planning, environmental responsibility, and regulatory alignment. Laboratories are adopting assays that reduce waste (through optimized pack sizes and streamlined consumables) and minimize repeat runs. As regulatory frameworks tighten—such as ongoing IVDR implementation in the EU and quality systems expectations worldwide—buyers increasingly prioritize suppliers who can support documentation, traceability, and reliable performance across the product lifecycle.
Taizhou Cowingene Biotech Co.,Ltd. positions its RSV offering with a forward‑looking approach: designing assays for efficient logistics, supporting standard instrumentation to extend asset life, and prioritizing materials that help reduce environmental footprint where feasible. Market trends also favor flexible testing menus—e.g., RSV stand‑alone detection or inclusion within broader respiratory workflows. With respiratory syncytial virus RNA PCR remaining central to hospital preparedness and public health monitoring, partners look to manufacturers who demonstrate resilience in supply, ongoing technical support, and a roadmap that aligns with emerging clinical and operational needs.
Conclusion on respiratory syncytial virus by pcr,respiratory syncytial virus rna pcr,rsv pcr positive from Taizhou Cowingene Biotech Co.,Ltd.
In modern in vitro diagnosis, respiratory syncytial virus by PCR offers the accuracy, speed, and operational reliability required by clinical laboratories and healthcare networks. Taizhou Cowingene Biotech Co.,Ltd. delivers an RSV RT‑PCR solution that supports consistent results—helping teams interpret RSV PCR positive or negative outcomes with confidence and scale testing when demand rises. For B2B decision makers, this translates into dependable performance, streamlined workflows, and a partner committed to quality and service.
Contact us: email: info@cowingene.com
Visit our website: https://www.cowingene.com

Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







