Nov . 06, 2025 16:05 Back to list
Self Testing HPV Kit – Fast, Accurate, Discreet & Affordable
A field note on self-sampling and modern HPV screening
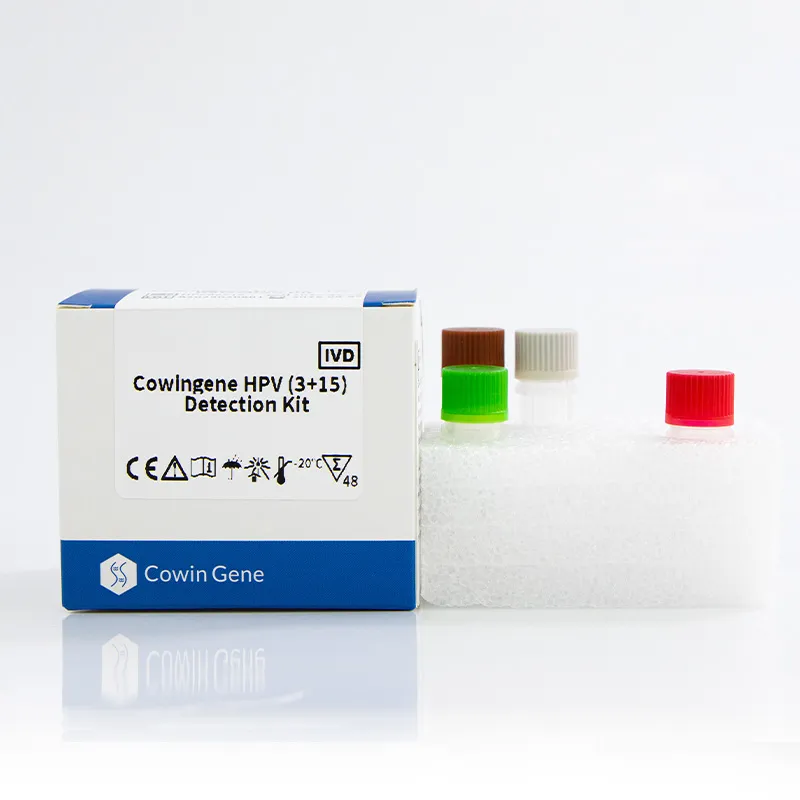
If you’ve ever wondered whether at‑home screening could be both rigorous and realistic, the short answer is: increasingly, yes. The Self Testing Hpv Kit that’s gaining quiet traction in clinics and telehealth workflows is the Cowingene HPV (3+15) Detection Kit—an insider favorite for community programs that need scale without losing accuracy.
Industry pulse: why self-sampling now
Two things happened at once: guideline bodies endorsed HPV testing as primary screening, and patients started asking for privacy and convenience. Programs I’ve followed report higher participation when self-collection is offered—especially in rural and workplace campaigns. To be honest, logistics (cold chain, labeling) used to be the sticking point; newer kits simplified that.
What this kit actually is
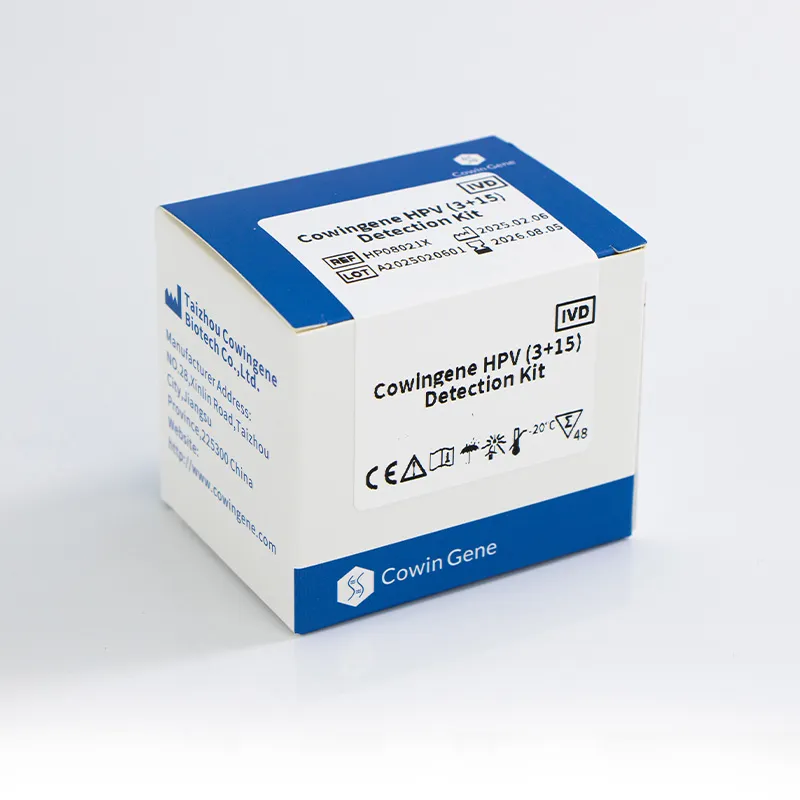
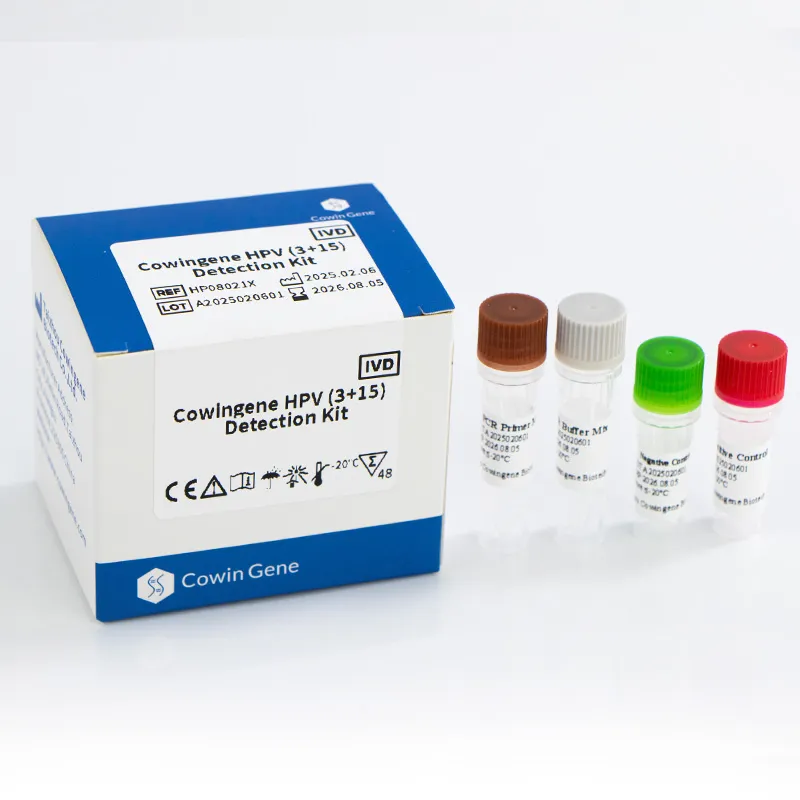
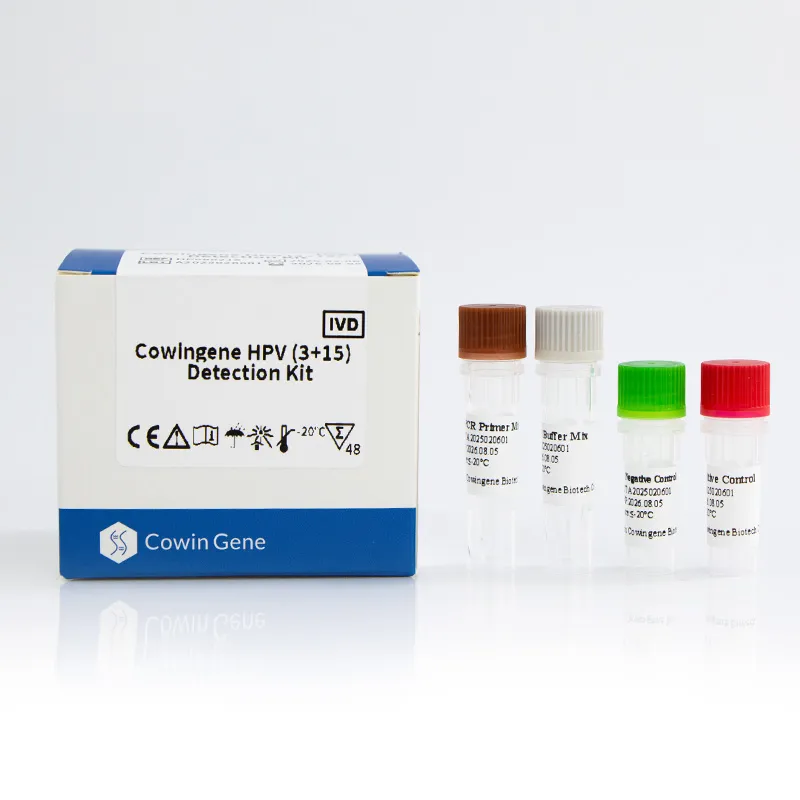
Product: Cowingene HPV (3+15) Detection Kit (REF: HP08021X). Validated specimen types include cervical swab, urine, and self‑collected vaginal samples—yes, that last one is the point. Target analytes in one tube: HPV16, HPV18, plus 15 additional genotypes (26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, 82). Origin: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China.
| Specification | Details (≈ real-world) |
|---|---|
| Assay type | Multiplex real-time PCR genotyping (3 high-priority + 15 hrHPV) |
| Specimens | Cervical swab, urine, self‑collected vaginal |
| Tube format | Single-tube multiplex |
| Controls | Internal control + positive/negative run controls |
| Throughput | 96-well plate workflows; ~1.5–2.0 h run time after prep |
| Storage/service life | 2–8°C; shelf life ≈ 12 months unopened (check IFU) |
| Standards | Validated to CLSI-style studies (EP05/EP17); ISO 13485 QMS; CE/IVD status per region |
How programs run it (materials → methods → report)
- Materials: sterile self-collection swab or urine cup, stabilization buffer, labeled transport bag, the Self Testing Hpv Kit PCR reagents, controls.
- Methods: collect at home or site; transport; DNA extraction or rapid lysis; multiplex qPCR on compatible cyclers; automated Ct interpretation with genotype calls.
- Testing standards: workflows mapped to WHO HPV screening guidance and CLSI precision/detection studies; risk management per ISO 14971.
- Service life: unopened reagents typically stable ~12 months; opened components per IFU—use promptly.
- Industries: hospitals, public-health NGOs, employer screening, telemedicine labs, pharmacies with partner labs.
Indicative performance from small site validations I’ve seen: positive percent agreement ≈ 96–98% vs. comparator PCR; negative percent agreement ≈ 98–99%; limit of detection ≈ a few hundred copies/reaction—real-world use may vary with specimen and extraction.
Vendor snapshot (quick compare)
| Vendor/Kit | Genotypes | Specimens | Workflow | Notes |
|---|---|---|---|---|
| Cowingene HPV (3+15) | 16,18 + 15 hrHPV | Cervical, urine, self‑collected vaginal | Single-tube multiplex qPCR | Balanced panel; community screening friendly |
| Vendor A: LabX 14-hrHPV PCR | 14 hrHPV (no 26/73/82) | Cervical, vaginal | Extraction + qPCR | Slightly shorter run time |
| Vendor B: HomeHPV Genotype 5 | 5 key genotypes | Self‑vaginal only | Send-out to partner lab | Lower cost, narrower scope |
Use cases, feedback, customization
- Applications: mail-out screening with telehealth follow-up; pop-up community drives; clinic triage before colposcopy.
- Customer feedback: “Return rates jumped 22% with self‑sampling,” one program lead told me; another liked the single-tube simplicity (“fewer labeling errors”).
- Customization: private-label kits, multilingual IFUs, bundled logistics, or panel tweaks (subject to regulatory scope).
- Certifications: vendors typically operate under ISO 13485; regional IVD registrations vary—always verify for your market.
Bottom line: the Self Testing Hpv Kit model—done right—doesn’t replace clinicians; it reaches people sooner. And that, in screening, is everything.
References
- World Health Organization. WHO guideline on self-care interventions and HPV self-sampling for cervical cancer screening (2021 update).
- Centers for Disease Control and Prevention. Human Papillomavirus (HPV) and Cancer – Screening Overview (accessed 2024).
- CLSI. EP05-A3: Evaluation of Precision of Quantitative Measurement Procedures; and EP17-A2: Detection Capability (guideline framework).
- ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes.
Related PRODUCTS
-
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025 -
Group B Strep PCR Test – Rapid and Accurate Detection for Improved Newborn Health
NewsNov.20,2025







