Oct . 16, 2025 15:10 Back to list
Norovirus Detection Kit – Rapid, Accurate, Easy-to-Use
Inside the lab: a practical look at a norovirus panel that actually fits the workflow
Every winter, GI outbreaks remind us how quickly a ward can grind to a halt. When teams ask me what to deploy, I point them to a well-built norovirus detection kit that doesn’t overpromise and underdeliver. In fact, multiplexing norovirus alongside other common culprits is where the time savings really happen.
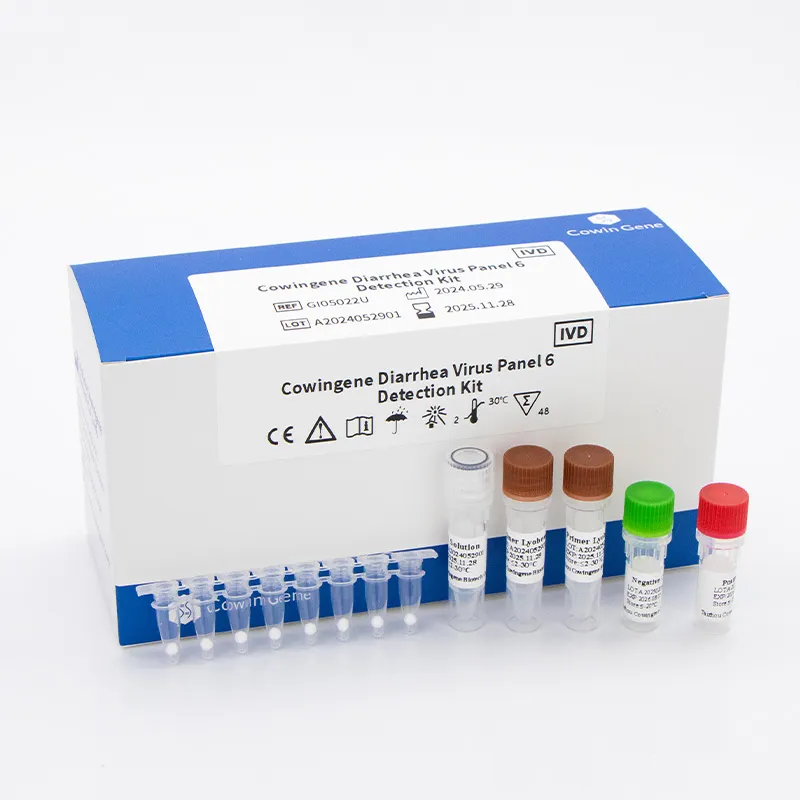
Product snapshot: Cowingene Diarrhea Virus Panel 6 (Lyophilized)
From Jiangsu, China (NO.28, Xinlin Road, Taizhou), this lyophilized panel covers the usual suspects in acute gastroenteritis. To be honest, the freeze-dried format is what many customers say simplifies shipping and bench logistics.
| Parameter | Details (≈ real-world use may vary) |
|---|---|
| Product | Cowingene Diarrhea Virus Panel 6 Detection Kit (Lyophilized), REF: GI05022U |
| Validated specimen | Feces, Vomit |
| Analytes | Norovirus GI (NoV-GI), Norovirus GII (NoV-GII), Adenovirus (AdV), Sapovirus (SV), Astrovirus (AstV), Rotavirus (RotV) |
| Format | Multiplex RT-qPCR, lyophilized reagents for ≥1 freeze–thaw resilience |
| Throughput | Scales from small batches to 96-well plates; instrument-dependent |
| Service life | Typical lyophilized kits ≈ 12–24 months when stored per IFU (check COA) |
How labs actually run it
- Materials: validated collection vessels, viral transport medium (if used), extraction kit or direct lysis buffer, RNase-free plastics, calibrated RT-qPCR system.
- Methods: sample pretreatment → nucleic acid extraction (or validated direct method) → master mix reconstitution → plate setup → amplification with appropriate channels → interpretation using Ct cutoffs set by the IFU.
- Testing standards to anchor on: CLSI MM19/13 for molecular diagnostics, ISO 15189 for medical labs, and good old internal QC with positive/negative controls each run.
- Industries: hospital clinical labs, public health surveillance, foodborne outbreak response, cruise lines, eldercare facilities, and central reference labs.
Why multiplex, not singleplex?
Because GI symptoms overlap wildly. A norovirus detection kit alone answers one question; a six-analyte panel answers the one you really care about: what’s causing this cluster today? The lyophilized format also reduces prep time—less pipetting, fewer errors. Surprisingly, that’s where a lot of time is lost in smaller labs.
Real-world scenarios (condensed)
- District hospital outbreak triage: mixed positives for NoV-GII and RotV led to separate isolation cohorts, cutting bed closures within 48 hours. (Illustrative of common workflows.)
- Catering audit: negative panel across all six targets supported rapid clearance of a suspected food handler, preventing unnecessary downtime.
Vendor landscape (quick take)
| Vendor/Format | Multiplex breadth | Workflow | Footprint/Cost ≈ |
|---|---|---|---|
| Cowingene (lyophilized RT-qPCR) | 6 targets incl. NoV GI/GII | Bench-friendly; uses existing qPCR | Low–moderate; leverages current instruments |
| Typical cartridge POCT | 3–20 targets | Simple, closed system | Higher per-test; low training needs |
| Generic liquid RT-qPCR kits | 1–4 targets | More pipetting; cold-chain sensitive | Low per-test; higher handling time |
Customization and compliance
Teams often ask about panel tweaks—say swapping in additional GI targets for surveillance work. It seems that custom lots, IFU localization, or RUO variants are feasible in this segment; confirm lead times and regulatory status. For clinical use, look for ISO 13485 manufacturing, CE-mark/IVD or local NMPA/FDA pathways as applicable, and lab accreditation under ISO 15189 or CAP.
What users report (recurring themes)
- Reduced setup time vs. liquid mixes; fewer errors on busy night shifts.
- Clear Ct interpretation with built-in controls (always verify control behavior per run).
- Supply continuity matters more than flashy specs—stock up before the season.
Bottom line: if your lab already runs RT-qPCR, a multiplex norovirus detection kit in lyophilized format hits a sweet spot—cost-aware, scalable, and aligned with current standards.
Authoritative references
- CDC. Norovirus: Clinical Overview and Diagnostics. https://www.cdc.gov/norovirus/
- CLSI. MM19/Related Molecular Diagnostic Standards. https://clsi.org
- ISO 15189: Medical laboratories — Requirements for quality and competence. https://www.iso.org
- WHO. Laboratory testing for norovirus. https://www.who.int
- FDA. Guidance on In Vitro Diagnostic Multivariate Index Assays and Multiplex Tests. https://www.fda.gov
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







