Oct . 23, 2025 16:45 Back to list
Real Time PCR HBV DNA Test – Fast, Sensitive, Accurate
A field-side look at Real Time Pcr Hbv Dna for blood-borne viruses
If you work in a blood bank or an ID lab, you’ve watched nucleic-acid testing evolve from “special test” to critical infrastructure. To be honest, Real Time Pcr Hbv Dna screening today is as much about workflow reliability and regulatory fit as raw sensitivity. That’s where the Cowingene HBV/HCV/HIV Detection Kit (NATBox) has been getting buzz—quietly, from hands-on users rather than billboards.
Industry trends, briefly
Three things are reshaping NAT: multiplex panels (one tube, several targets), decentralized but connected instruments, and standards-first operations (ISO 15189, CLSI MM-methods). Labs say they want kits that keep Ct stability across lots, play nicely with their extractors, and don’t make IT cry.
What the kit is
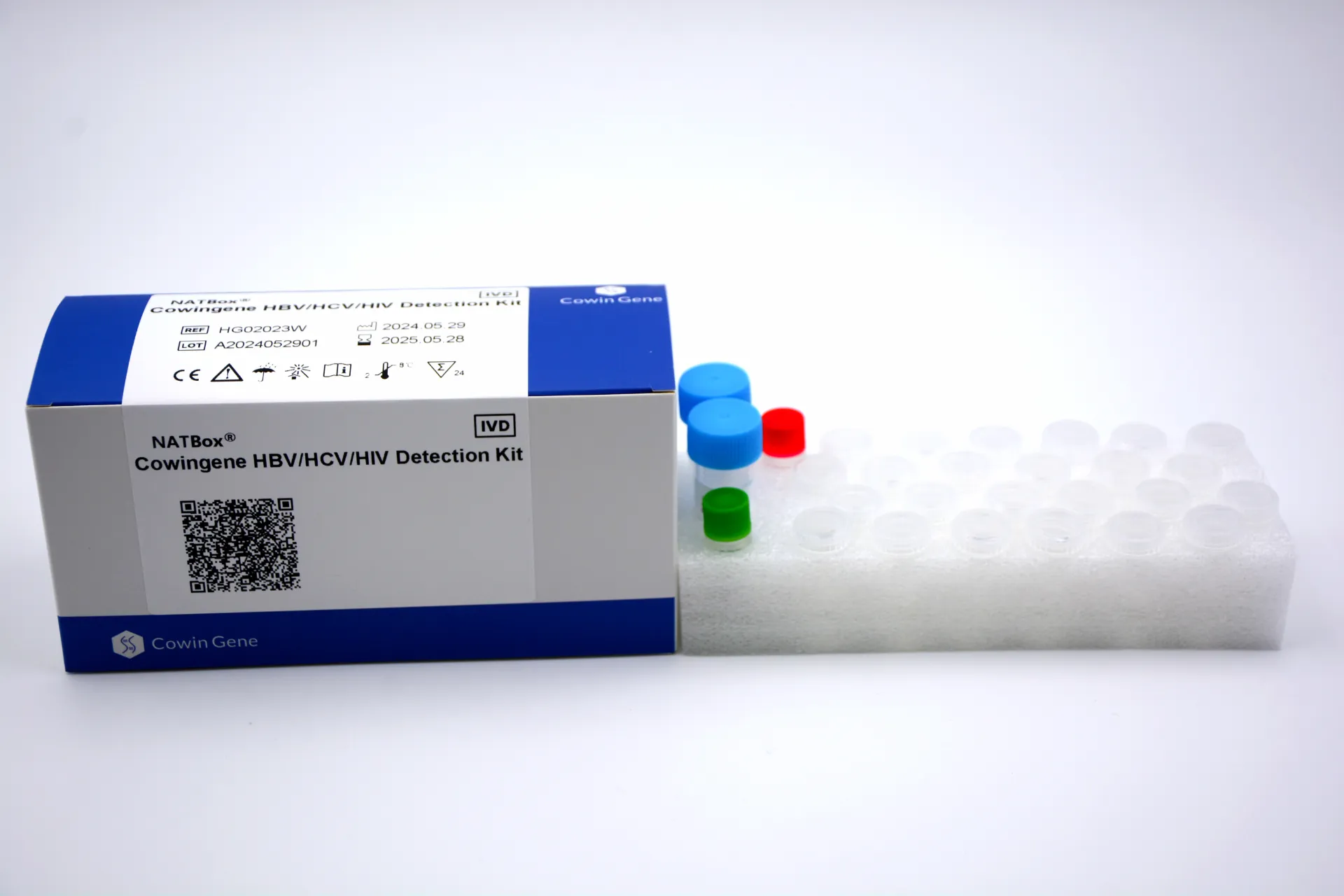
Cowingene’s NATBox kit detects HBV, HCV, and HIV in plasma/serum using real-time PCR chemistry. Validated specimen types: plasma, serum. Origin: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China. It’s pitched for donor screening and clinical monitoring—different workflows, same backbone chemistry.
| Parameter | Details (≈, real-world use may vary) |
|---|---|
| Product | Cowingene HBV/HCV/HIV Detection Kit (NATBox) |
| Targets | HBV DNA, HCV RNA, HIV RNA (multiplex) |
| Specimens | Plasma, Serum |
| Run time | ≈ 90–120 min including extraction |
| Storage / Service life | -20°C; shelf life ≈ 12–18 months (check IFU/label) |
| Controls | Internal extraction control; positive/negative controls recommended per CLSI |
| Certification | Region-specific regulatory status; verify CE-IVD/IVDR or local approvals |
| Compatibility | Common open qPCR cyclers and extraction systems; confirm channels/layout |

Process flow (materials and methods)
- Materials: EDTA plasma/serum, extraction kit (manual or automated), NATBox reagents, PCR plates/tubes, calibrated pipettes, aerosol tips.
- Method: Nucleic-acid extraction → master mix setup (HBV/HCV/HIV multiplex + internal control) → thermocycling → Ct analysis with target-specific thresholds.
- Testing standards: Follow CLSI MM03 for molecular ID assays; verify lot performance with positive/negative controls; EQA participation recommended.
- QC expectations: Positive control Ct within lot mean ±2 SD; NTC undetermined; internal control Ct around 25–32 depending on matrix.
- Service life: Kit within labeled expiry; instrument lifecycle ≈ 5–7 years with annual PM.
- Industries: Blood centers, hospital labs, public health screening, CROs, transplant programs.
Applications and advantages
Use it for donor screening (minipool or ID-NAT), baseline staging, or treatment monitoring where policies allow. Advantages many customers mention: steady Ct drift across lots, sensible multiplex that doesn’t punish low HBV viremia, and clean internal control signaling. Actually, the straightforward interpretation is the thing techs like most.
| Vendor/Platform | Targets | Throughput | Hands-on | Notes |
|---|---|---|---|---|
| Cowingene NATBox | HBV/HCV/HIV | Mid (open systems) | Low–moderate | Flexible, cost-conscious; open extractor compatibility |
| Roche cobas | Multiple viral assays | High | Low | Highly automated, larger footprint |
| Abbott Alinity m | Multiple viral assays | High | Low | Random-access convenience |
| Qiagen artus (open) | HBV/HCV/HIV (assay-dependent) | Mid | Moderate | Open, modular workflows |
Customization, support, and feedback
Cowingene offers OEM-style options—panel configuration, pack sizes, language IFU, and LIS-friendly result exports. It seems that regional teams can align to local standards faster than expected. A lab manager told me, “We wanted tighter pooling guidance for Real Time Pcr Hbv Dna workflows, and the update came quick.” Nice when it happens.
Mini case notes
- Regional blood bank: moved from ELISA+confirm to NAT-first; reported smoother donor deferral decisions and fewer repeats, especially for low-titer Real Time Pcr Hbv Dna positives.
- Transplant center: multiplex run trimmed TAT on urgent screens; IT appreciated CSV outputs for audit trails.
Standards and verification tips
Anchor your validation to ISO 15189 and CLSI MM03. Use WHO HBV/HCV international standards where available, and document precision (CLSI EP05) plus qualitative agreement (EP12). Keep periodic EQA; many programs now include multiplex NAT challenges.
Citations
- WHO. Guidelines for hepatitis B and C testing. World Health Organization.
- CLSI. MM03: Molecular Diagnostic Methods for Infectious Diseases—Approved Guideline.
- ISO 15189:2022 Medical laboratories—Requirements for quality and competence.
- EASL. Clinical Practice Guidelines on HBV (latest update).
- CDC. Blood Safety and Laboratory Testing resources for HBV/HCV/HIV NAT.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







