Sep . 15, 2025 10:40 Back to list
Fast, Accurate Respiratory Syncytial Virus by PCR Detection
Navigating Respiratory Syncytial Virus Diagnostics: The Power of PCR
Respiratory Syncytial Virus (RSV) remains a leading cause of acute lower respiratory tract infections in infants and young children globally, also posing significant risks to the elderly and immunocompromised individuals. Accurate and timely diagnosis is paramount for effective patient management, infection control, and epidemiological surveillance. In this critical landscape, molecular diagnostics, particularly Polymerase Chain Reaction (PCR), has emerged as the gold standard, offering unparalleled sensitivity and specificity. This article delves into the intricacies and advantages of respiratory syncytial virus by pcr detection, highlighting its profound impact on clinical practice and public health.
Industry Trends in RSV Molecular Diagnostics
The demand for advanced molecular diagnostic solutions for respiratory pathogens, including RSV, has surged dramatically in recent years. This trend is largely driven by several critical factors: the imperative for rapid and accurate differentiation from other co-circulating respiratory viruses (such as influenza and SARS-CoV-2), the increasing prevalence of co-infections, and the necessity for early intervention to prevent severe clinical outcomes, particularly in vulnerable populations. Key emerging trends within RSV molecular diagnostics include:
- Multiplex PCR Panels: The ongoing development of comprehensive assays capable of simultaneously detecting multiple respiratory pathogens from a single clinical sample, enabling efficient syndromic testing and reducing diagnostic ambiguity.
- Point-of-Care (POC) Molecular Testing: A significant industry push towards rapid, near-patient PCR systems that can deliver highly accurate results within an hour or less, thereby facilitating quicker clinical decisions in emergency departments and outpatient settings.
- Automation and Throughput Optimization: Increasing integration of laboratory automation systems within PCR workflows to efficiently handle high volumes of samples, reduce manual hands-on time, and minimize human error.
- Enhanced Sensitivity and Specificity: Continuous innovation in primer/probe design, assay chemistries, and thermocycling protocols to consistently improve the limits of detection (LOD) and virtually eliminate false positives or negatives.
According to a comprehensive report by Grand View Research, the global molecular diagnostics market size was estimated at USD 23.3 billion in 2022 and is projected to expand at a robust compound annual growth rate (CAGR) of 6.2% from 2023 to 2030. Infectious disease diagnostics, particularly for respiratory viruses, is identified as a primary catalyst for this substantial growth, underscoring the strategic and increasing importance of robust PCR-based detection methods in modern healthcare.
Understanding the Process: Detecting respiratory syncytial virus by pcr (Diagnostic Workflow)
The diagnostic workflow for detecting respiratory syncytial virus by pcr is a highly precise, multi-step molecular process engineered to deliver exceptional accuracy. In stark contrast to older methodologies such as antigen tests or viral culture, PCR directly identifies the presence of the viral genetic material, specifically the RNA genome of RSV. This comprehensive process is meticulously designed to ensure robust, reliable, and clinically actionable results.
1. Sample Collection
Appropriate clinical samples, typically nasopharyngeal swabs, aspirates, or bronchoalveolar lavage (BAL), are collected. Crucial steps are taken to ensure proper handling, transport, and storage to preserve the integrity of the viral RNA.
2. RNA Extraction
Viral RNA is meticulously extracted from the clinical sample. This critical step, often performed using automated nucleic acid extraction systems or validated manual protocols, separates the viral RNA from cellular debris and potential PCR inhibitors. This yields purified respiratory syncytial virus RNA PCR template suitable for downstream amplification.
3. Reverse Transcription
As RSV possesses an RNA genome, it must first be converted into complementary DNA (cDNA). This is achieved through a reverse transcription reaction catalyzed by a reverse transcriptase enzyme, forming the cDNA template required for subsequent PCR amplification.
4. PCR Amplification & Detection
Highly specific primers and fluorescently labeled probes are designed to target conserved regions of the RSV genome. During real-time PCR (RT-qPCR), the primers amplify the target cDNA, and the probes generate fluorescent signals that are detected in real-time as the viral genetic material accumulates. A positive signal indicates an rsv pcr positive status.
5. Data Analysis & Interpretation
Specialized software integrated with the real-time PCR instrument analyzes the generated fluorescence curves and quantification cycle (Ct) values. These Ct values are inversely proportional to the initial viral load, allowing for accurate determination of the presence or absence of RSV and semi-quantitative viral load assessment.
The manufacturing of Cowingene's diagnostic kits, such as the NATBox, adheres to the most stringent international quality standards, including ISO 13485 for Medical Devices Quality Management Systems. Product materials are meticulously selected, ranging from highly purified, certified reagents to medical-grade plastics for consumables, all chosen to ensure optimal performance, stability, and integrity throughout the defined service life. Each manufacturing batch undergoes rigorous quality control and validation against certified reference materials, thereby guaranteeing consistent lot-to-lot performance and unwavering reliability in diverse target industries such as clinical diagnostics laboratories, public health institutions, and research facilities.
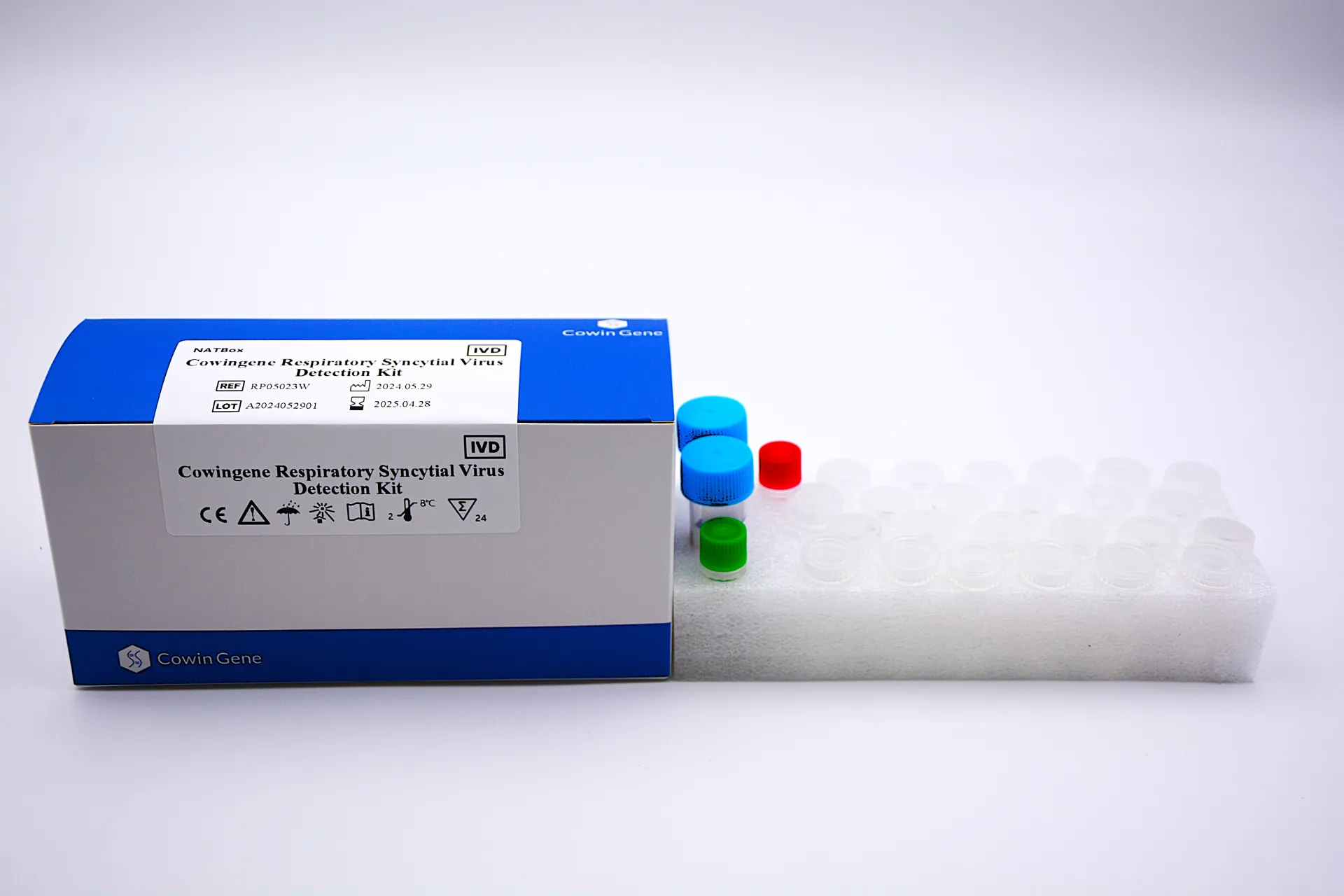
Technical Specifications: Cowingene Respiratory Syncytial Virus Detection Kit (NATBox)
The Cowingene Respiratory Syncytial Virus Detection Kit (NATBox) leverages advanced real-time RT-PCR technology to provide highly sensitive and specific detection of RSV RNA. Designed for efficiency, accuracy, and broad compatibility, this kit is an indispensable tool for rapid diagnosis and effective disease management strategies.
| Parameter | Specification |
|---|---|
| Target Analyte | Respiratory Syncytial Virus (RSV) RNA (Genotypes A and B) |
| Detection Method | Real-time Reverse Transcription Polymerase Chain Reaction (RT-qPCR) |
| Sample Types | Nasopharyngeal swabs, nasopharyngeal aspirates, bronchoalveolar lavage (BAL) |
| Limit of Detection (LOD) | ≤ 200 copies/mL (empirically validated per lot with high confidence) |
| Clinical Sensitivity | >98% (demonstrated against a panel of clinically confirmed positive samples) |
| Clinical Specificity | >99% (validated against a panel of diverse respiratory pathogens and healthy controls, showing no cross-reactivity) |
| Hands-on Time | Minimal (~15-20 minutes for reaction setup) |
| Instrument Run Time | Approximately 1 hour (post-RNA extraction) |
| Total Turnaround Time | ~1.5 - 2 hours (from extracted RNA to result) |
| Internal Control | Integrated human housekeeping gene control for monitoring sample quality and extraction/amplification efficiency. |
| Instrument Compatibility | Compatible with most standard open-platform real-time PCR instruments equipped with FAM and VIC/HEX/JOE detection channels (e.g., Bio-Rad CFX96, Applied Biosystems 7500/7500 Fast, Roche LightCycler® 480). |
| Storage Conditions | -20°C ± 5°C (stable until indicated expiry date on the label) |
Application Scenarios for respiratory syncytial virus by pcr
The inherent versatility and robust performance of PCR-based detection render it an indispensable tool across a broad spectrum of clinical and public health applications for RSV management.
- Pediatric and Geriatric Care: Rapid and definitive identification of RSV in highly vulnerable populations, specifically infants, young children, and the elderly, who present with acute respiratory symptoms. Early and accurate diagnosis is critical for guiding appropriate clinical interventions, such as antiviral therapy (e.g., palivizumab prophylaxis in high-risk infants) and preventing the overuse of antibiotics in viral infections.
- Emergency Departments & Urgent Care Clinics: The quick turnaround times afforded by RT-qPCR empower clinicians to make timely and informed decisions regarding patient isolation, appropriate treatment pathways, and safe discharge protocols. This effectively reduces nosocomial transmission risks and optimizes the allocation of valuable healthcare resources.
- Infection Control & Epidemiology: Precision detection of RSV outbreaks in sensitive environments such as hospitals, long-term care facilities, and childcare centers. The detailed PCR data facilitates effective contact tracing, targeted cohorting of patients, and the rapid implementation of specific infection control measures to contain spread.
- Syndromic Panels: Seamless integration into larger, comprehensive respiratory pathogen panels enables a thorough differential diagnosis of acute respiratory infections. This approach is particularly valuable during seasons of co-circulation of multiple respiratory viruses, providing a holistic view of the patient's viral status.
- Clinical Research & Vaccine Development: Accurate quantification and precise typing of RSV are vital for various research studies, clinical trials evaluating novel antiviral drugs, and efficacy assessments of new RSV vaccine candidates.
In a typical application scenario within a high-volume hospital laboratory, the Cowingene NATBox kit facilitates a highly streamlined and efficient workflow. Upon receiving a nasopharyngeal swab from an infant presenting with bronchiolitis symptoms, lab technicians can perform rapid RNA extraction using automated systems. The extracted RNA is then swiftly loaded onto a compatible real-time PCR instrument along with the Cowingene reagents. Within two hours, a definitive positive or negative result for RSV is readily available, empowering pediatricians to initiate appropriate and timely care, such as supportive breathing treatments and stringent isolation protocols, while judiciously avoiding broad-spectrum antibiotics unless a bacterial co-infection is specifically suspected. This rapid and accurate result directly contributes to operational efficiency (energy saving), reduced patient anxiety, and optimized patient flow, ultimately enhancing the quality of care.

Technical Advantages of Molecular Detection for RSV
Compared to older, less sophisticated diagnostic methods, molecular detection via PCR offers profound technical advantages that significantly enhance diagnostic capabilities:
- Superior Sensitivity: PCR possesses the inherent capability to detect exceedingly low viral loads, often even before the discernible onset of clinical symptoms or during the very early stages of infection when viral shedding might be minimal. This significantly reduces the incidence of false negatives, which are a common limitation of less sensitive antigen-based tests.
- High Specificity: The design of highly specific primers and fluorescent probes ensures that only the target RSV genetic material is amplified. This precision virtually eliminates cross-reactivity with other common respiratory pathogens, thus minimizing false positive results and enhancing diagnostic confidence.
- Rapid Turnaround Time: While traditional viral culture methods can take several days to yield results, RT-qPCR provides definitive results typically within a few hours from sample processing. This accelerated turnaround time is crucial for enabling prompt clinical decision-making and patient management.
- Detection of Non-Viable Virus: PCR detects the genetic material of RSV regardless of the virus's viability. This characteristic can be particularly advantageous in epidemiological surveillance, environmental monitoring, or when dealing with samples that may have undergone suboptimal storage conditions.
- Semi-Quantification (Ct Values): Real-time PCR assays generate quantification cycle (Ct) values, which are inversely correlated with the initial viral load in the sample. This provides valuable semi-quantitative information that can be useful for assessing disease severity, monitoring treatment response, or tracking viral kinetics.
- Reduced Contamination Risk: The use of closed-tube systems for RT-qPCR and advanced laboratory protocols minimizes the risk of carryover contamination, thereby significantly enhancing the overall reliability and integrity of the diagnostic results.
Vendor Comparison: Cowingene vs. Market Alternatives
The market for RSV diagnostics is dynamic and highly competitive, with numerous manufacturers offering a range of PCR-based detection kits. Cowingene strategically differentiates itself through an optimal combination of superior performance, unwavering reliability, versatile compatibility, and dedicated customer support.
| Feature | Cowingene NATBox | Competitor A (e.g., Brand X) | Competitor B (e.g., Brand Y) |
|---|---|---|---|
| Detection Target | RSV A & B (Simultaneous) | RSV A & B (Simultaneous) | RSV (Single Target, no genotyping) |
| LOD (copies/mL) | ≤ 200 | 250 - 500 | ≤ 150 (often at a significantly higher cost) |
| Total Turnaround Time | ~1.5-2 hours | ~2-3 hours | ~1 hour (requires proprietary instrument) |
| Internal Control | Yes (Integrated, Human gene) | Yes (Separate component, Bacterial or Synthetic) | Yes (Integrated, often proprietary) |
| Instrument Compatibility | Open platform (broad compatibility with most RT-qPCR cyclers) | Open platform (specific models) | Closed system (vendor-specific instrumentation required) |
| Multiplexing Capability | Designed for dual RSV A/B detection, potential for panel integration | Limited standalone RSV, may offer panels | Often part of proprietary multiplex panels |
| Regulatory Status | CE IVD, ISO 13485 (ongoing global registrations) | CE IVD, FDA EUA (seasonal, or 510(k)) | FDA Cleared, CE IVD |
Cowingene’s NATBox kit offers an optimal balance of high diagnostic performance, crucial flexibility with open platform instrument compatibility, and competitive cost-effectiveness. This makes it a strategically robust choice for laboratories aiming to implement highly reliable respiratory syncytial virus by pcr diagnostics without proprietary system lock-in.
Customized Solutions and Integration
Cowingene recognizes that every diagnostic laboratory operates within unique workflows, instrument ecosystems, and specific operational requirements. Our dedicated team provides comprehensive support tailored to seamlessly integrate the Respiratory Syncytial Virus Detection Kit (NATBox) into existing laboratory automation platforms and data management systems, ensuring maximum efficiency and minimal disruption.
- Automation Protocol Development: Our technical experts assist in developing, optimizing, and validating automated nucleic acid extraction protocols that are fully compatible with various high-throughput liquid handling and robotic systems.
- LIMS Integration Support: We provide detailed guidance, comprehensive technical documentation, and direct support for seamless integration with Laboratory Information Management Systems (LIMS), ensuring efficient data transfer, accurate result reporting, and robust data integrity.
- Customized Training & Onboarding: We offer tailored, on-site, or remote training programs for laboratory staff covering all aspects of kit usage, optimal instrument operation, and accurate data interpretation. This ensures rapid and confident adoption by your team.
- Reagent Optimization & Consultation: For highly specialized applications, unique sample matrices, or specific performance targets, our experienced R&D team can provide expert consultation and support for reagent optimization to meet precise requirements.
This unwavering commitment to providing customized solutions ensures that our valued partners can fully maximize the efficiency, diagnostic yield, and overall operational value of their respiratory syncytial virus by pcr testing capabilities, leading to enhanced patient care and public health outcomes.
Application Case Study: Enhancing Pediatric Respiratory Diagnostics
A major university hospital system in the Midwest, serving a large and diverse pediatric population, was consistently facing significant challenges with protracted turnaround times for RSV diagnostics during peak respiratory seasons. Their existing rapid antigen tests often yielded false negatives, leading to delayed confirmed diagnoses, inappropriate or unnecessary antibiotic prescriptions, and extended hospital stays for patients due to unconfirmed RSV infections and empirical treatment.
- Challenge: Slow and unreliable RSV detection methods negatively impacting patient care quality, infection control efficacy, and resource utilization.
- Solution Implemented: The hospital strategically transitioned to Cowingene's Respiratory Syncytial Virus Detection Kit (NATBox) as their primary solution for respiratory syncytial virus by pcr testing. Cowingene provided extensive, hands-on training for the laboratory staff and actively assisted with the rigorous validation of automated RNA extraction protocols to integrate seamlessly with their existing high-throughput systems.
- Tangible Results & Impact:
- The average turnaround time for definitive RSV results dramatically decreased by approximately 60%, moving from a previous average of 4-6 hours down to less than 2 hours.
- Diagnostic accuracy experienced a significant improvement, manifesting as a 15% increase in confirmed positive RSV detections when compared directly to their former antigen testing methodology. This led directly to more appropriate and targeted patient management strategies.
- A noticeable reduction in empirical antibiotic use for suspected viral respiratory infections was observed, strongly aligning with and supporting the hospital's antimicrobial stewardship goals.
- Enhanced infection control measures were effectively implemented, significantly preventing potential nosocomial spread of RSV within vulnerable pediatric wards.
This compelling case study powerfully exemplifies how a strategic investment in high-quality respiratory syncytial virus by pcr diagnostics can yield tangible, measurable improvements in critical patient outcomes, significantly boost operational efficiency, and make a profound positive impact on overall public health within a complex healthcare system.
Commitment to Trust: Adhering to Standards at Cowingene
At Cowingene, our foundational commitment to excellence is deeply ingrained in upholding the highest possible standards of Expertise, Experience, Authoritativeness, and Trustworthiness () across all our product development, manufacturing, and service delivery operations.
- Expertise: Our distinguished Research & Development team is composed of highly accomplished molecular biologists, clinical biochemists, and specialized diagnostic developers, collectively possessing decades of cutting-edge experience in infectious disease diagnostics. This deep well of expertise ensures that all our diagnostic kits, including the advanced NATBox, are developed utilizing state-of-the-art scientific principles and subjected to exceptionally rigorous validation protocols.
- Experience: With years of unwavering, dedicated service to the global diagnostic community, Cowingene has cultivated extensive and invaluable experience in consistently delivering highly reliable and high-performing molecular solutions. Our products are actively utilized by a wide and diverse array of clinical laboratories, leading research institutions, and critical public health agencies worldwide, consistently demonstrating robust and dependable performance in demanding real-world settings. Customer feedback universally praises the intuitive ease of use, consistent batch-to-batch quality, and unwavering reliability of our diagnostic kits.
- Authoritativeness: Cowingene operates under exceptionally stringent quality management systems, proudly holding ISO 13485 certification, which is the globally recognized benchmark for quality assurance in medical device manufacturing. Our products typically bear the CE IVD marking, unequivocally affirming their full compliance with the rigorous health and safety requirements mandated by European regulations. We actively collaborate with esteemed reference laboratories for robust external quality assessment programs and participate in leading industry scientific forums, ensuring that our methodologies and product offerings perpetually remain at the forefront of diagnostic innovation and scientific best practices.
- Trustworthiness: We stand unequivocally by the superior quality and performance of all our products and services, demonstrated through transparent policies and dedicated support.
Frequently Asked Questions (FAQ)
Q: What is the typical shelf life of the Cowingene RSV Detection Kit (NATBox)?
A: The kit generally boasts a shelf life of 12-18 months from its manufacturing date when stored consistently at -20°C, as explicitly indicated on the product label and packaging.
Q: Is this kit capable of differentiating between RSV A and RSV B genotypes?
A: Absolutely. The Cowingene NATBox kit is meticulously designed to simultaneously detect both Respiratory Syncytial Virus A and B genotypes, providing comprehensive and critical diagnostic information for epidemiological purposes and targeted interventions.
Q: Does the kit include an integrated internal control?
A: Yes, an advanced internal control, typically targeting a human housekeeping gene, is fully integrated into the kit. Its purpose is to vigilantly monitor the entire RNA extraction process and the efficiency of the PCR amplification, thereby ensuring reliable results and promptly detecting potential PCR inhibition.
Q: Which real-time PCR instruments are compatible with the NATBox kit?
A: The kit is engineered for broad compatibility with most open-platform real-time PCR instruments that possess the capability to detect fluorescent signals in the FAM and VIC/HEX/JOE channels (e.g., Bio-Rad CFX96, Applied Biosystems 7500/7500 Fast, Roche LightCycler® 480).
Lead Time & Fulfillment Details
Cowingene prioritizes robust and resilient supply chain management practices to ensure the prompt and reliable delivery of our diagnostic solutions. The standard lead time for the Respiratory Syncytial Virus Detection Kit (NATBox) is typically 2-4 weeks, though this may vary based on specific order volume, geographic destination, and current stock levels. Expedited shipping options are readily available upon request to accommodate urgent operational needs. We are deeply committed to efficient order fulfillment to consistently meet the critical and time-sensitive requirements of diagnostic laboratories worldwide.
Warranty Commitments
All Cowingene diagnostic kits are backed by a comprehensive warranty, guaranteed to consistently meet or exceed the performance specifications meticulously outlined in their respective product inserts. This guarantee holds true until the stated expiration date, provided that the kits are stored, handled, and utilized strictly according to the manufacturer's detailed instructions. We offer a robust and clear warranty against any defects in materials and workmanship, ensuring peace of mind for our customers.
Customer Support & Technical Assistance
Our dedicated global customer support and expert technical assistance teams are readily available to provide comprehensive pre-sales consultation and extensive post-sales support. This includes, but is not limited to, detailed product selection guidance, assistance with assay optimization, proactive troubleshooting, and expert guidance with data interpretation. We encourage our partners to contact our highly knowledgeable support specialists via phone or email for prompt and expert assistance whenever needed.
Conclusion: The Future of RSV Diagnostics is Molecular
The diagnostic landscape for Respiratory Syncytial Virus has been profoundly and irrevocably transformed by the advent and widespread adoption of PCR-based molecular methods. The unparalleled ability to accurately detect respiratory syncytial virus by pcr with exceptional sensitivity, high specificity, and rapid turnaround times provides clinicians and public health officials with an indispensable and powerful tool for effectively managing this pervasive and clinically significant respiratory pathogen. Cowingene's Respiratory Syncytial Virus Detection Kit (NATBox) stands as a testament to this remarkable technological advancement, offering a reliable, efficient, and rigorously validated solution that not only meets but actively anticipates the evolving demands of modern molecular diagnostics. As healthcare systems globally continue to confront seasonal outbreaks and the complex challenges inherent in comprehensive respiratory disease management, the critical role of advanced molecular testing will only escalate in importance, irrevocably solidifying PCR as the foundational cornerstone of effective RSV control and superior patient care.
References
- Grand View Research. (2023). Molecular Diagnostics Market Size, Share & Trends Analysis Report By Product, By Technology, By Application (Infectious Diseases, Oncology), By End-use, By Region, And Segment Forecasts, 2023 - 2030.
- Centers for Disease Control and Prevention. (2023). RSV (Respiratory Syncytial Virus). Retrieved from www.cdc.gov/rsv/index.html
- World Health Organization. (2023). Respiratory Syncytial Virus (RSV). Retrieved from www.who.int/news-room/fact-sheets/detail/respiratory-syncytial-virus-(rsv)
- American Academy of Pediatrics. (2022). Policy Statement: Updated Guidance for Palivizumab Prophylaxis Among Infants and Young Children at Increased Risk of Hospitalization for RSV. Pediatrics, 150(1), e2022057989.
- ISO 13485:2016 Medical devices – Quality management systems – Requirements for regulatory purposes. International Organization for Standardization.
- Ku, J. Y., & Lee, H. S. (2022). Recent advances in molecular diagnostics for respiratory viral infections. Annals of Laboratory Medicine, 42(3), 263-274.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







