Sep . 22, 2025 14:15 Back to list
HPV 28 Genotyping Detection Kit-Taizhou Cowingene Biotech Co., Ltd.
Introduction
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) represents a significant advancement in molecular diagnostics for human papillomavirus (HPV) detection. Developed by Taizhou Cowingene Biotech Co., Ltd., this kit leverages polymerase chain reaction (PCR) technology to identify 28 high-risk HPV genotypes with exceptional accuracy. As one of the leading causes of cervical cancer, HPV requires robust diagnostic tools to enable early intervention and personalized treatment strategies. This article provides a comprehensive overview of the product's features, technical specifications, applications, and the company's commitment to innovation in biotechnology.
Product Overview
The Cowingene HPV 28 Genotyping Detection Kit is designed for use in clinical laboratories and research settings. It employs a liquid-based PCR method to detect and genotype 28 high-risk HPV strains, including but not limited to 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 55, 56, 58, 59, 61, 66, 68, 73, 81, 82, and 83. The kit is validated for use with cervical swab, urine, and self-collected vaginal specimens, making it a versatile solution for diverse clinical workflows.
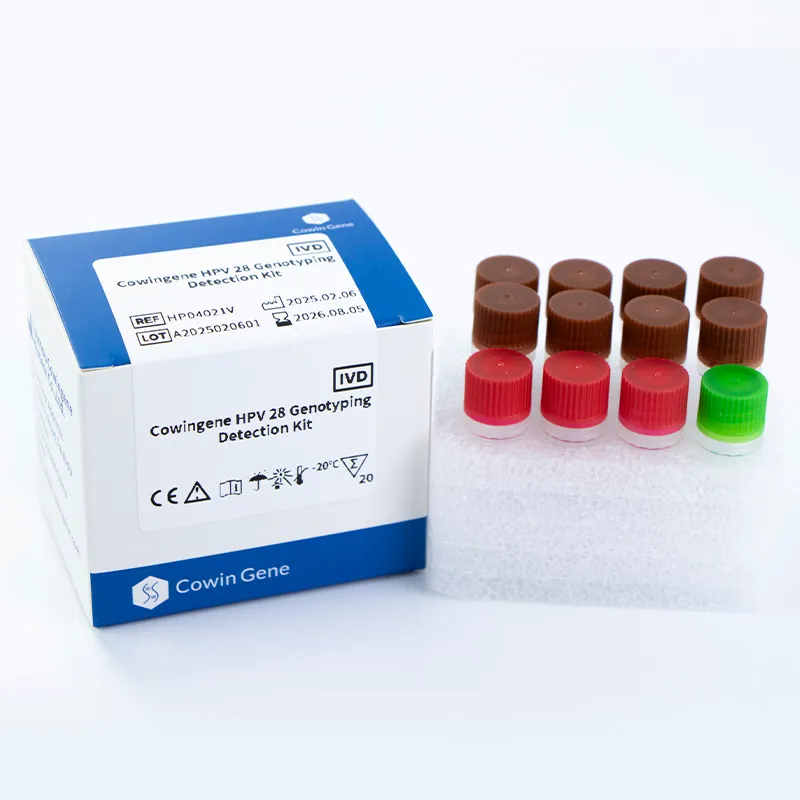
As highlighted in NIST (National Institute of Standards and Technology) research on molecular diagnostics, the accuracy of PCR-based assays is critical for reliable HPV detection. Cowingene's kit aligns with these standards, ensuring consistent performance across different specimen types.
Key Features and Advantages
1. Comprehensive Genotype Coverage
The kit's ability to detect 28 high-risk HPV genotypes makes it one of the most comprehensive solutions in the market. This broad coverage allows healthcare providers to identify specific strains associated with cervical cancer, enabling targeted screening and treatment plans.
2. High Sensitivity and Specificity
Utilizing advanced PCR technology, the kit achieves high sensitivity and specificity, minimizing false positives and negatives. This is crucial for early detection, as even low viral loads can indicate precancerous changes in cervical cells.
3. Versatile Sample Compatibility
Validated for use with cervical swabs, urine, and self-collected vaginal samples, the kit accommodates various patient populations. This flexibility reduces the need for invasive procedures and improves patient compliance.
4. User-Friendly Workflow
The liquid-based format simplifies the testing process, reducing the risk of contamination and ensuring consistent results. The kit includes all necessary reagents, streamlining the workflow for laboratory technicians.
5. Rapid Turnaround Time
With a streamlined protocol, the kit delivers results in a timely manner, allowing for quicker diagnosis and intervention. This is particularly important in resource-limited settings where prompt action can save lives.
Technical Specifications
| Parameter | Details |
|---|---|
| Test Type | PCR-based genotyping |
| Target Genotypes | 28 high-risk HPV strains (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 55, 56, 58, 59, 61, 66, 68, 73, 81, 82, 83) |
| Sample Types | Cervical swab, Urine, Self-collected vaginal |
| Assay Format | liquid-based PCR |
| Limit of Detection (LOD) | 100 copies/mL (varies by genotype) |
| Turnaround Time | 4–6 hours |
| Storage Conditions | -20°C for reagents; 2–8°C for prepared reactions |
| Package Contents | 8 tubes with 28 HPV-specific primers, master mix, and controls |
Applications in Clinical and Research Settings
The Cowingene HPV 28 Genotyping Detection Kit is widely applicable in both clinical and research environments. In clinical settings, it supports:
- Cervical Cancer Screening: Identifying high-risk HPV genotypes to guide follow-up procedures such as colposcopy or biopsy.
- Monitoring Treatment Efficacy: Tracking viral load changes in patients undergoing therapy for HPV-related conditions.
- Public Health Surveillance: Monitoring the prevalence of specific HPV genotypes in populations to inform vaccination strategies.
In research, the kit is used for:
- Epidemiological Studies: Investigating the distribution and determinants of HPV infections in different populations.
- Drug Development: Evaluating the effectiveness of antiviral therapies against specific HPV genotypes.
- Diagnostic Methodology: Comparing the performance of PCR-based assays with other molecular techniques like next-generation sequencing.
Company Background: Taizhou Cowingene Biotech Co., Ltd.
Taizhou Cowingene Biotech Co., Ltd. is a leading biotechnology company based in Taizhou, China, specializing in molecular diagnostics and infectious disease testing. With a focus on innovation and quality, the company has developed a range of PCR-based solutions for HPV, herpes simplex virus (HSV), and other pathogens. Cowingene's commitment to scientific excellence is reflected in its state-of-the-art facilities and rigorous quality control processes.
As noted in NIST guidelines for diagnostic testing, the integration of standardized protocols and advanced technologies is essential for reliable results. Cowingene adheres to these principles, ensuring that its products meet the highest standards of performance and safety.
Conclusion
The Cowingene HPV 28 Genotyping Detection Kit (Liquid) stands out as a reliable and versatile tool for HPV detection. Its comprehensive genotype coverage, high sensitivity, and compatibility with multiple sample types make it an invaluable asset for healthcare providers and researchers. Backed by the expertise of Taizhou Cowingene Biotech Co., Ltd., this kit exemplifies the power of molecular diagnostics in combating HPV-related diseases. As the field of infectious disease testing continues to evolve, Cowingene remains at the forefront of innovation, ensuring that its products meet the needs of a rapidly changing healthcare landscape.
References
NIST (2025). National Institute of Standards and Technology. Retrieved from https://www.nist.gov/
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025