Sep . 22, 2025 13:10 Back to list
N. Gonorrhoeae PCR: Rapid, Accurate Detection Solution
n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection is a key solution in the Biomedicine industry, specifically within in vitro diagnosis and Molecular Diagnostics. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
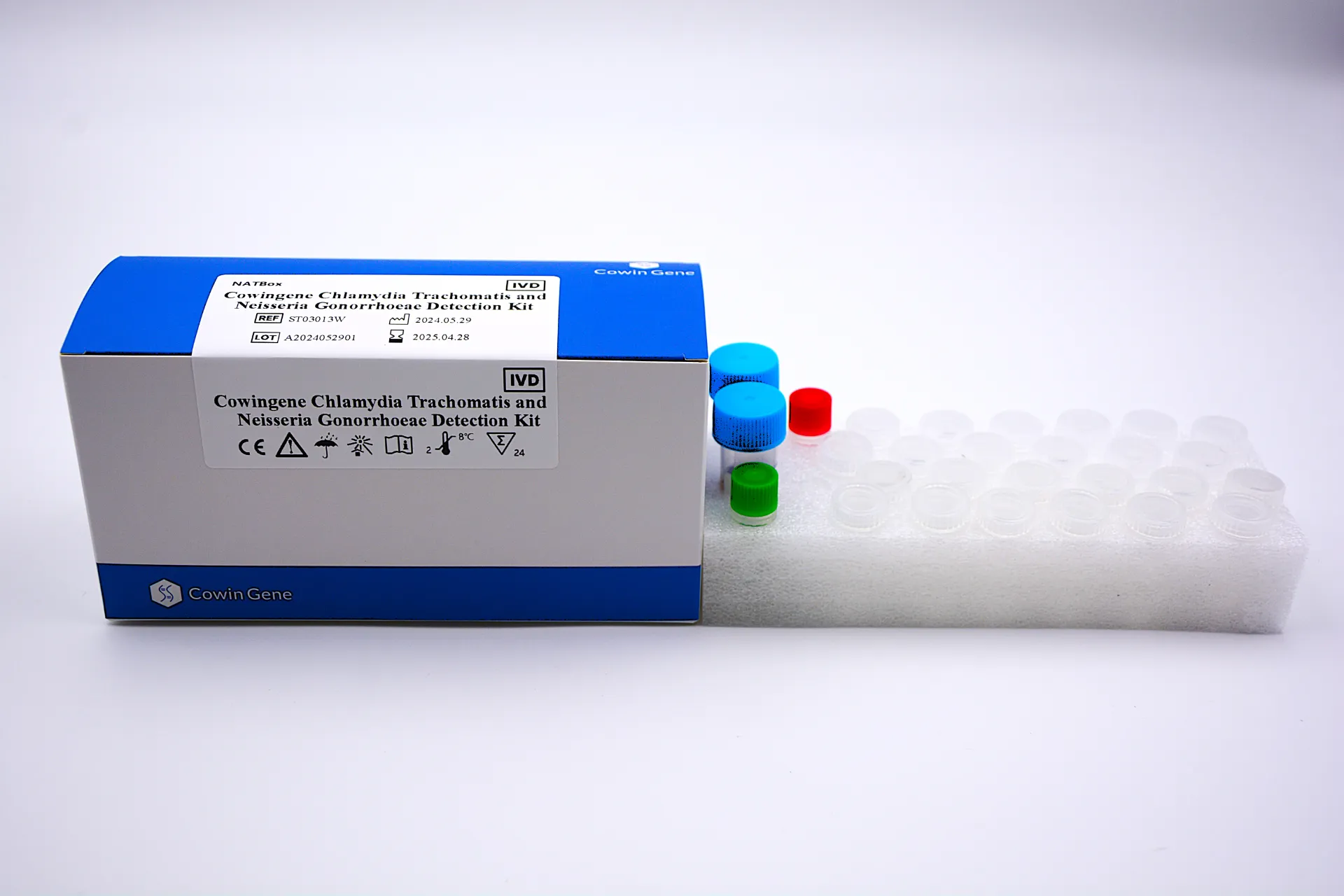
Table of Contents
- n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection Overview
- Benefits & Use Cases of n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection in Molecular Diagnostics
- Cost, Maintenance & User Experience
- Sustainability & Market Trends in Biomedicine
- Conclusion on n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection from Taizhou Cowingene Biotech Co.,Ltd.
n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection Overview
In the rapidly evolving field of Molecular Diagnostics, the accurate and timely identification of infectious agents is paramount. Taizhou Cowingene Biotech Co.,Ltd. stands at the forefront, offering advanced solutions for critical diagnostic needs, including reliable methods for n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection. This innovative product addresses the challenge of diagnosing sexually transmitted infections (STIs) caused by Neisseria gonorrhoeae and Chlamydia trachomatis, two of the most prevalent bacterial STIs globally. Our diagnostic kits leverage the power of Polymerase Chain Reaction (PCR) technology, offering superior sensitivity and specificity compared to traditional culture or immunoassay methods. This PCR-based approach allows for the direct detection of bacterial DNA, even at low pathogen concentrations, significantly improving diagnostic accuracy. For laboratories and healthcare providers engaged in in vitro diagnosis, selecting a robust and precise tool for both Chlamydia trachomatis and Neisseria gonorrhoeae detection is crucial for effective patient management and public health initiatives. Taizhou Cowingene Biotech Co.,Ltd. is recognized as a reliable manufacturer committed to providing high-quality diagnostic products that meet stringent industry standards.Benefits & Use Cases of n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection in Molecular Diagnostics
The capabilities of our n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection solutions are specifically tailored to meet the demanding requirements of modern Molecular Diagnostics. These assays offer a multiplexing advantage, allowing for the simultaneous detection of both Chlamydia trachomatis and Neisseria gonorrhoeae from a single patient sample. This not only streamlines workflows in clinical laboratories but also provides comprehensive results faster, a critical factor in preventing further transmission of STIs. Key features include high analytical sensitivity, enabling the detection of infections earlier than conventional methods, and exceptional specificity to minimize false positives. Our PCR solutions are designed for ease of use, integrating seamlessly into existing lab automation systems, which is a significant competitive advantage for busy diagnostic centers. For decision-makers in B2B settings, investing in such advanced chlamydia trachomatis neisseria gonorrhoeae pcr technology means fewer retests, quicker treatment initiation, and ultimately, better patient outcomes. Taizhou Cowingene Biotech Co.,Ltd. brings extensive expertise to this sector, ensuring that our N. gonorrhoeae PCR and Neisseria gonorrhoeae detection products are both innovative and dependable, reflecting our commitment to advancing in vitro diagnostics.Cost, Maintenance & User Experience
When considering investments in Molecular Diagnostics, B2B decision-makers prioritize not only performance but also the total cost of ownership (TCO), durability, and user experience. Taizhou Cowingene Biotech Co.,Ltd.'s solutions for n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection are engineered for long-term reliability and cost-effectiveness. The upfront investment in high-quality PCR reagents and kits translates into significant long-term ROI due to reduced labor costs, fewer repeat tests, and enhanced diagnostic confidence. Our kits are designed for minimal maintenance, featuring robust components and straightforward protocols that require less hands-on time from laboratory personnel. This user-centric design improves efficiency and reduces the likelihood of human error, which is critical in an in vitro diagnosis setting. Feedback from customers in the diagnostics sector consistently highlights the kits' ease of integration, clear instructions, and consistent performance, making the process of Chlamydia trachomatis Neisseria gonorrhoeae PCR both reliable and manageable. This dedication to durability and a positive user experience underscores our commitment to providing value beyond just the initial purchase, ensuring that our Neisseria gonorrhoeae detection solutions truly empower diagnostic laboratories.Sustainability & Market Trends in Biomedicine
The landscape of Biomedicine, particularly in vitro diagnosis, is continually shaped by evolving market trends, regulatory standards, and a growing emphasis on sustainability. As a forward-thinking company, Taizhou Cowingene Biotech Co.,Ltd. is acutely aware of these dynamics. Our commitment to sustainable practices extends from our manufacturing processes to the lifecycle of our diagnostic products, including those for n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection. We strive to minimize waste, optimize resource consumption, and ensure compliance with all relevant environmental regulations. The global market for STI diagnostics is projected for significant growth, driven by increasing awareness, improved access to healthcare, and the need for more accurate and rapid testing. Regulatory bodies increasingly demand stringent performance standards, which our Neisseria gonorrhoeae detection assays consistently meet and exceed. By offering cutting-edge molecular diagnostics that are both effective and responsibly produced, Taizhou Cowingene Biotech Co.,Ltd. positions itself as an eco-conscious partner. We contribute to public health while adhering to the highest ethical and environmental standards, aligning with the long-term vision of our B2B clients in the biomedical industry.Conclusion on n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection from Taizhou Cowingene Biotech Co.,Ltd.
The ability to accurately and efficiently detect infectious diseases is fundamental to public health. Taizhou Cowingene Biotech Co.,Ltd.'s solutions for n gonorrhoeae pcr,chlamydia trachomatis neisseria gonorrhoeae pcr,neisseria gonorrhoeae detection represent a pinnacle of precision and reliability in the Molecular Diagnostics field. Offering unparalleled sensitivity and specificity, these PCR assays empower laboratories with the tools necessary for rapid and confident diagnosis of Neisseria gonorrhoeae and Chlamydia trachomatis. For B2B decision-makers in in vitro diagnosis and Biomedicine, partnering with Taizhou Cowingene Biotech Co.,Ltd. means investing in a product that delivers exceptional performance, operational efficiency, and a sustainable future. We pride ourselves on our reputation for innovation and quality.Ready to enhance your diagnostic capabilities?
- Contact us: email: info@cowingene.com
- Visit our website: https://www.cowingene.com
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







