Nov . 04, 2025 13:43 Zurück zur Liste
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
Respiratory Panel Test For is a key solution in the healthcare industry, specifically within Medical Devices and Diagnostics and in vitro diagnosis. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
Inhaltsverzeichnis
- Respiratory Panel Test For Overview
- Benefits & Use Cases of Respiratory Panel Test For in in vitro diagnosis
- Kosten, Wartung und Benutzererfahrung
- Sustainability & Market Trends in healthcare
- Conclusion on Respiratory Panel Test For from Taizhou Cowingene Biotech Co.,Ltd.
Respiratory Panel Test For Overview
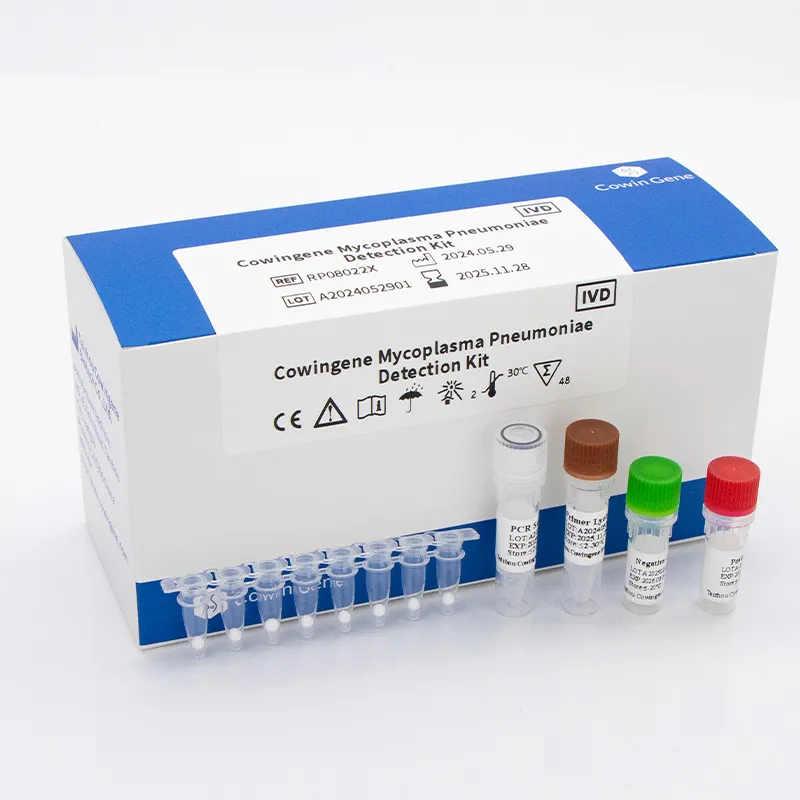
For laboratories, hospitals, and integrated delivery networks, a modern Respiratory Panel Test For rapid detection of common pathogens is foundational to clinical decision-making and operational efficiency. Powered by respiratory pathogen panel PCR methodologies, these assays enable fast, sensitive, and specific identification of bacterial and viral targets from upper respiratory specimens. Within this landscape, Taizhou Cowingene Biotech Co.,Ltd. offers targeted solutions such as its Mycoplasma pneumoniae Detection Kit (lyophilized), designed to fit seamlessly into real-time PCR workflows used by clinical and reference laboratories. The lyophilized format supports streamlined logistics, consistent performance, and simplified preparation compared with liquid reagents.
- Product relevance: Enables quick, reliable detection of respiratory pathogens—vital during peak seasons and outbreaks—while supporting evidence-based triage and antimicrobial stewardship.
- Technical background: Real-time PCR chemistry with built-in controls helps assure run validity; compatible with common thermocyclers and standard nucleic acid extraction methods.
- Reliable manufacturer: Taizhou Cowingene Biotech Co.,Ltd. is a trusted provider in the in vitro diagnosis market, delivering robust assay design, dependable supply continuity, and responsive technical support.
Benefits & Use Cases of Respiratory Panel Test For in in vitro diagnosis
In vitro diagnosis workflows increasingly rely on a respiratory pathogen panel to inform fast, syndromic decisions across emergency departments, urgent care, pediatrics, and ICU settings. A Respiratory Panel Test For targeted organisms like Mycoplasma pneumoniae complements broader respiratory pathogen panel PCR strategies, allowing labs to triage between multiplex syndromic panels and focused, cost-efficient singleplex or small panel assays as clinical questions evolve. This flexible testing ladder improves throughput and helps match test intensity with clinical need.
- Applications: Hospital laboratories, third-party reference labs, and public health networks use targeted PCR kits to confirm specific pathogens following a positive syndromic screen or to run reflex testing during seasonal surges.
- Competitive advantages: Lyophilized, ready-to-use reagents reduce hands-on time and logistical complexity; high analytical sensitivity/specificity supports confident results; compatibility with widely used real-time PCR platforms protects prior capital investments.
- Cowingene expertise: Taizhou Cowingene Biotech Co.,Ltd. combines assay optimization experience with practical deployment support—protocols, training, and responsive troubleshooting—to help labs scale capacity while maintaining quality.
Kosten, Wartung und Benutzererfahrung
For B2B decision makers, the total cost of ownership matters as much as assay performance. A Respiratory Panel Test For targeted organisms in a lyophilized format can lower logistics costs by reducing cold-chain dependence, cut waste through longer reagent stability, and minimize repeat runs via integrated controls and consistent lot-to-lot performance. Because Cowingene’s kits work with common extraction systems and PCR instruments, organizations leverage existing assets rather than purchasing specialized hardware—accelerating ROI and shortening validation timelines.
- TCO and durability: Ready-to-use, shelf-stable reagent formats streamline inventory management and reduce per-test overhead, especially during seasonal demand spikes.
- User feedback: Laboratory managers frequently note simpler onboarding, fewer manual steps, and reliable Ct consistency across runs—features that translate into smoother operations and predictable turnaround times in the Medical Devices and Diagnostics environment.
Sustainability & Market Trends in healthcare
Syndromic respiratory testing continues to expand as health systems push for data-driven antimicrobial stewardship and preparedness for seasonal surges or new variants. The shift toward scalable respiratory pathogen panel PCR strategies—balancing multiplex panels with targeted assays—aligns with budget discipline and resilience planning. Regulatory expectations are also elevating documentation, traceability, and performance verification across the IVD supply chain. In this context, sustainability is no longer optional: reduced cold-chain shipments, compact packaging, and efficient lab workflows all contribute to lower environmental impact and cost.
- Eco-conscious operations: Lyophilized formats can decrease dry-ice use and shipping weight, while well-designed kit components reduce consumable waste and storage footprint.
- Forward-thinking partner: Taizhou Cowingene Biotech Co.,Ltd. operates with quality systems aligned to global IVD expectations and invests in product designs that support sustainable, dependable supply for hospitals and reference labs worldwide.
Conclusion on Respiratory Panel Test For from Taizhou Cowingene Biotech Co.,Ltd.
A well-executed Respiratory Panel Test For targeted pathogens complements broader syndromic strategies, enabling faster clinical decisions, efficient resource use, and scalable operations across the Medical Devices and Diagnostics value chain. Taizhou Cowingene Biotech Co.,Ltd. pairs strong assay performance with practical benefits—lyophilized convenience, instrument compatibility, and responsive support—making it a reliable partner for in vitro diagnosis. Ready to strengthen your respiratory testing portfolio? Contact us: email: info@cowingene.com — Visit our website: https://www.cowingene.com
Verwandt PRODUKTE
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
NachrichtNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
NachrichtNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
NachrichtNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
NachrichtNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
NachrichtNov.17,2025



























