Sep . 02, 2025 05:20 Back to list
Respiratory Panel Test For Rapid Pathogen Identification
Industry Trends in Respiratory Pathogen Testing
The landscape of infectious disease diagnostics has been profoundly reshaped by advancements in molecular biology, particularly in the realm of respiratory illnesses. The demand for rapid, accurate, and comprehensive diagnostic tools, such as the respiratory panel test for various pathogens, has surged, driven by global health challenges and the need for effective public health management. Current trends indicate a strong shift towards multiplex PCR assays, which enable simultaneous detection of multiple viral and bacterial targets from a single patient sample. This approach significantly reduces turnaround time, optimizes resource utilization, and facilitates more targeted treatment strategies, thereby improving patient outcomes and curbing antimicrobial resistance.
Emerging technologies, including next-generation sequencing (NGS) and CRISPR-based diagnostics, are beginning to complement traditional PCR methods, offering even higher levels of sensitivity and specificity, alongside the potential for rapid identification of novel or uncommon pathogens. The market is also seeing increased integration of automated systems, moving from manual laboratory procedures to fully automated platforms capable of high-throughput analysis. This automation is critical for large-scale testing during outbreaks and for enhancing reproducibility and reducing human error. Furthermore, point-of-care (POC) respiratory panel test for solutions are gaining traction, promising rapid results outside of centralized laboratories, which is particularly beneficial in remote areas or emergency settings.
Manufacturing Process Flow of a Respiratory Panel Test Kit
The manufacturing of a sophisticated diagnostic product, such as a respiratory panel test for multiple pathogens, involves a meticulously controlled process to ensure product efficacy, reliability, and compliance with stringent regulatory standards like ISO 13485 (Medical Devices – Quality Management Systems). This process typically begins with the careful selection and synthesis of high-quality raw materials.
Key Process Steps:
- Oligonucleotide Synthesis & Purification: High-purity primers and probes, specifically designed to target conserved regions of respiratory pathogen genomes (e.g., SARS-CoV-2, Influenza A/B, RSV, Mycoplasma pneumoniae), are synthesized using phosphoramidite chemistry. These are then rigorously purified to remove truncated sequences and other impurities that could affect assay sensitivity and specificity.
- Enzyme Production & Validation: Recombinant enzymes, such as reverse transcriptase and DNA polymerase (often hot-start variants for improved specificity), are produced in controlled bioreactor environments. These enzymes undergo extensive functional validation to ensure optimal activity and stability in the final assay formulation.
- Buffer Formulation & QC: Proprietary buffer systems are prepared to maintain optimal pH, ionic strength, and enzyme activity, crucial for PCR amplification efficiency. Each batch of buffer components is subjected to strict quality control, including sterility and endotoxin testing.
- Reagent Blending & Dispensing: All purified oligonucleotides, enzymes, and buffers are precisely blended under aseptic conditions to form the master mix. This master mix is then accurately dispensed into PCR tubes or plates using automated liquid handling systems, minimizing batch-to-batch variation. For lyophilized kits, like the Cowingene Mycoplasma Pneumoniae Detection Kit, this involves a subsequent lyophilization step.
- Lyophilization (Freeze-Drying): This critical process removes water from the dispensed reagents through sublimation, creating a stable, dry pellet. Lyophilization significantly extends shelf life, reduces cold chain requirements, and simplifies shipping and storage. The process is tightly controlled, monitoring temperature, pressure, and time.
- Assembly & Packaging: The lyophilized reagents are sealed and packaged with desiccants in moisture-barrier pouches or plates, often with an inert gas environment, to maintain stability. Kits are assembled with control templates, internal controls, and detailed instructions for use (IFU).
- Final Quality Control & Validation: Each manufacturing lot undergoes comprehensive QC, including analytical specificity, analytical sensitivity (LoD), inclusivity, exclusivity, precision, and reproducibility testing using a panel of characterized clinical samples and reference strains. Compliance with ISO and other relevant regulatory standards (e.g., FDA, CE-IVD) is confirmed before release.
These rigorous processes ensure that the final respiratory panel test for pathogens delivers consistent, high-performance results, crucial for clinical diagnostics in industries ranging from public health and hospitals to reference laboratories.
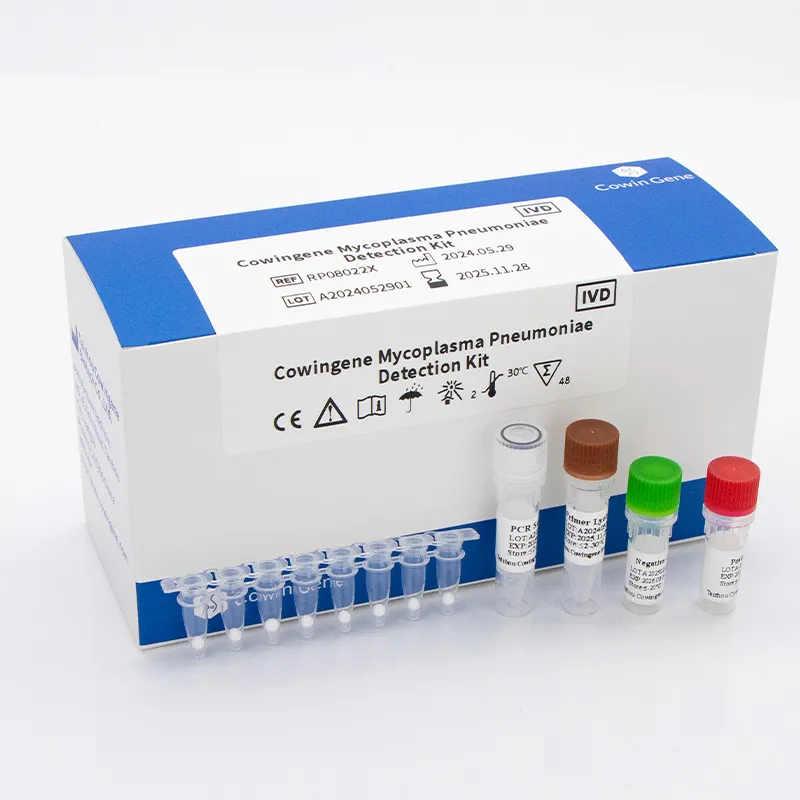
Technical Specifications and Parameters
Modern respiratory panel test for systems are characterized by a set of critical technical specifications that dictate their utility and performance in clinical settings. These include analytical sensitivity, specificity, limit of detection (LoD), dynamic range, and compatibility with various sample types and PCR instruments. For instance, the Cowingene Mycoplasma Pneumoniae Detection Kit (Lyophilized) exemplifies precision engineering in molecular diagnostics.
Cowingene Mycoplasma Pneumoniae Detection Kit (Lyophilized) - Key Specifications:
| Parameter | Specification |
|---|---|
| Target Pathogen | Mycoplasma Pneumoniae |
| Assay Type | Real-Time Quantitative PCR (qPCR) |
| Sample Type Compatibility | Nasopharyngeal swabs, throat swabs, bronchoalveolar lavage fluid (BALF), sputum |
| Limit of Detection (LoD) | < 500 copies/mL (varies by instrument) |
| Specificity | >99% (no cross-reactivity with common respiratory flora or other pathogens) |
| Sensitivity | >95% (compared to reference method) |
| Reaction Volume | 20 μL/reaction |
| Format | Lyophilized (ready-to-use beads) |
| Storage Conditions | 2-8°C (long-term), -20°C (after reconstitution) |
| Shelf Life | 12 months from manufacturing date |
The lyophilized format of such kits significantly enhances stability and simplifies logistics, eliminating the need for strict cold chain transport, which is a major advantage for global distribution and emergency response. This also extends the service life and ensures high quality when used in diverse environments.
Application Scenarios for Respiratory Panel Tests
The versatility and high performance of a respiratory panel test for multiple pathogens make it indispensable across a wide range of clinical and public health application scenarios. These tests play a crucial role in improving diagnostic accuracy, guiding appropriate treatment, and informing epidemiological surveillance.
- Hospital and Clinical Laboratories: Rapid and accurate diagnosis of respiratory infections (e.g., influenza, RSV, COVID-19, Mycoplasma pneumoniae, Chlamydia pneumoniae) in symptomatic patients to differentiate between viral and bacterial causes, enabling targeted therapy and reducing unnecessary antibiotic prescriptions. This is critical in managing severe acute respiratory infections (SARI) and guiding infection control measures.
- Emergency Departments: Expedited diagnosis for patients presenting with acute respiratory symptoms, allowing for quicker isolation of highly contagious individuals and optimizing patient flow, particularly during peak seasons for respiratory viruses.
- Public Health Surveillance: Monitoring the prevalence and spread of respiratory pathogens within communities, detecting emerging strains, and informing public health interventions and vaccination campaigns. High-throughput respiratory pathogen panel PCR tests are invaluable for this purpose.
- Immunocompromised Patients: Prompt identification of even subtle infections in vulnerable populations (e.g., transplant recipients, cancer patients, individuals with autoimmune diseases) where respiratory infections can lead to severe complications.
- Long-Term Care Facilities: Containing outbreaks by quickly identifying the causative agent, protecting vulnerable residents, and preventing widespread transmission.
- Research and Development: Supporting studies on respiratory disease epidemiology, pathogen evolution, and the development of new therapeutics and vaccines.
These applications underscore the critical role of advanced diagnostic panels in modern healthcare infrastructure, contributing to both individual patient care and broader public health initiatives.
Technical Advantages of Modern Respiratory Pathogen Panels
The evolution of respiratory pathogen panel PCR tests has introduced significant technical advantages over traditional diagnostic methods, making them indispensable in today's clinical and public health laboratories. These advantages translate directly into improved clinical outcomes and operational efficiency.
- Multiplexing Capability: Simultaneously detect multiple respiratory viruses and bacteria from a single patient sample. This comprehensive approach avoids the need for sequential testing, reducing turnaround time and providing a holistic view of potential co-infections.
- High Sensitivity and Specificity: Molecular assays, particularly qPCR, offer unparalleled sensitivity, capable of detecting even low viral or bacterial loads early in the infection course. High specificity minimizes false positives, ensuring reliable results.
- Rapid Turnaround Time (TAT): From sample to result, many modern systems can deliver answers within 1-3 hours, a critical factor for patient management, infection control, and outbreak response.
- Quantitative Results: Some panels provide quantitative viral/bacterial load data, which can be useful for monitoring disease progression, treatment response, and assessing infectivity.
- Reduced Sample Volume Requirements: Advanced molecular techniques allow for robust detection even from small sample volumes, making them suitable for pediatric or challenging sample collections.
- Automation Compatibility: Many panels are designed for integration with automated nucleic acid extraction and PCR platforms, reducing hands-on time, minimizing contamination risks, and enabling high-throughput processing.
- Lyophilized Reagents: As seen with the Cowingene kit, lyophilized reagents offer superior shelf-life, reduced storage requirements (often room temperature or refrigerated, not frozen), and simplified shipping, especially beneficial for global distribution and emergency stockpiling. This also simplifies preparation, reducing pipetting errors.
These advantages collectively empower healthcare providers with the tools needed to manage respiratory infections more effectively, leading to better patient care and more efficient healthcare system operations.
Vendor Comparison: Respiratory Pathogen Panels
Selecting the right respiratory pathogen panel involves evaluating various vendors based on assay breadth, instrument compatibility, turnaround time, cost-effectiveness, and regulatory approvals. Below is a comparative overview of typical offerings in the market, highlighting different approaches to a respiratory panel test for comprehensive pathogen detection.
Comparative Analysis of Respiratory Panel Test Offerings
| Feature/Vendor | Vendor A (e.g., BioFire FilmArray) | Vendor B (e.g., Luminex NxTAG) | Vendor C (e.g., Cowingene - single pathogen example, Mycoplasma pneumoniae) |
|---|---|---|---|
| Assay Type | Multiplex Nested PCR | Multiplex Real-Time PCR | Real-Time qPCR |
| Pathogens Detected (Typical Panel) | ~20 targets (viruses & bacteria) | ~20 targets (viruses & bacteria) | Mycoplasma Pneumoniae (specific) |
| Turnaround Time | ~1 hour | ~3 hours (batch processing) | ~1.5-2 hours |
| Required Instrumentation | Proprietary BioFire System | Luminex MAGPIX/FLEXMAP 3D | Standard Real-Time PCR cyclers (e.g., Applied Biosystems, Bio-Rad) |
| Automation Level | Fully automated (sample-in, result-out) | Automated extraction, semi-automated PCR | Manual extraction, automated PCR (lyophilized reagent simplifies) |
| Reagent Format | Integrated Pouch | Liquid Reagents | Lyophilized Beads |
| Cost per Test (Indicative) | Higher (premium for speed/automation) | Medium-High (batch efficiency) | Cost-effective (flexible platform) |
| Regulatory Approvals | FDA-cleared, CE-IVD | FDA-cleared, CE-IVD | CE-IVD (for example) |
This comparison highlights the trade-offs between highly integrated, rapid, and proprietary systems versus more flexible, platform-agnostic solutions. For specialized diagnostic needs, like detecting a single, specific pathogen such as Mycoplasma pneumoniae, a dedicated, cost-effective kit like Cowingene’s lyophilized option presents significant advantages, especially for laboratories with existing qPCR infrastructure.
Customized Solutions for Respiratory Diagnostics
Recognizing the diverse needs of different laboratories and healthcare systems, many diagnostic providers offer customized solutions for respiratory panel test for systems. These tailor-made approaches ensure that diagnostic capabilities align perfectly with specific clinical demands, regional epidemiological profiles, and budgetary constraints.
Customization options can include the development of bespoke panels targeting specific pathogens relevant to a particular geographic area or patient population (e.g., highly endemic viruses or bacteria). This could involve modifying existing panels by adding or removing certain targets, or developing entirely new assays from the ground up. Furthermore, solutions can be adapted for compatibility with a client's existing laboratory equipment, optimizing integration and reducing the need for new capital expenditure. This might involve adapting reagent formats, adjusting reaction volumes, or providing specific software integration support. For example, a reference laboratory might require a high-throughput automated system for screening thousands of samples, while a small regional hospital might need a simpler, rapid point-of-care solution. Providers often work closely with clients through a consultative process to define specific performance criteria, validate custom assays, and ensure regulatory compliance, such as adherence to ISO 17025 for testing and calibration laboratories, ensuring tailored solutions meet the highest standards of quality and performance.
Application Case Studies
Real-world applications demonstrate the impact and effectiveness of advanced respiratory panel test for solutions in clinical diagnostics and public health.
Case Study 1: Rapid Outbreak Response in a Pediatric Hospital
During a severe respiratory season, a major pediatric hospital faced an influx of children presenting with flu-like symptoms. Traditional single-target PCR assays were causing delays in diagnosis and patient isolation, leading to potential nosocomial transmission. The hospital implemented a multiplex respiratory pathogen panel system capable of detecting 15 common respiratory viruses and bacteria within 1.5 hours. This rapid diagnostic capability reduced the average time to diagnosis from 8 hours to 2 hours. This led to a 30% reduction in unnecessary antibiotic prescriptions, a 40% decrease in isolation bed days, and a significant improvement in hospital patient flow. The ability to quickly identify specific pathogens, including co-infections, allowed for tailored antiviral or antibacterial treatments and swift implementation of infection control measures, preventing further spread within the vulnerable pediatric population.
Case Study 2: Mycoplasma Pneumoniae Detection in a Community Outbreak
A community health clinic observed an unusual clustering of atypical pneumonia cases, particularly among school-aged children. Initial broad-panel respiratory tests were negative for common viral pathogens. Suspecting a bacterial etiology, they utilized the Cowingene Mycoplasma Pneumoniae Detection Kit (Lyophilized). Its high sensitivity and specificity confirmed Mycoplasma pneumoniae as the causative agent in over 70% of the cases within 2 hours of sample reception. The kit's lyophilized format simplified storage and handling, making it ideal for the smaller clinic's laboratory setup. This rapid and specific diagnosis enabled timely administration of appropriate macrolide antibiotics, leading to quick resolution of symptoms and preventing further community spread. The ease of use and reliable results of the specific respiratory panel test for Mycoplasma pneumoniae proved critical in managing this localized outbreak.
Frequently Asked Questions (FAQ)
- Q1: What is the typical turnaround time for a respiratory pathogen panel result?
- A1: Turnaround times vary significantly by system. Fully automated, sample-to-result systems can deliver results in as little as 1 hour, while more traditional batch-processed PCR panels may take 2-4 hours, not including sample preparation time. Our lyophilized kits are designed for efficient workflows, often yielding results within 1.5 to 2 hours post-extraction.
- Q2: How do lyophilized reagents improve the diagnostic process?
- A2: Lyophilized reagents, like those in the Cowingene Mycoplasma Pneumoniae Detection Kit, offer enhanced stability, allowing for storage at ambient or refrigerated temperatures (2-8°C) for extended periods, eliminating the need for strict cold chain logistics. They also simplify assay setup, reducing pipetting steps and potential for errors, leading to more consistent results and greater user convenience.
- Q3: What types of samples are compatible with respiratory panel tests?
- A3: Most respiratory panel tests are validated for use with a variety of upper and lower respiratory samples, including nasopharyngeal swabs, throat swabs, sputum, bronchoalveolar lavage (BAL) fluid, and nasopharyngeal aspirates/washes. Compatibility may vary slightly between different kits and manufacturers.
- Q4: Are these tests suitable for use in low-resource settings?
- A4: While advanced molecular diagnostics often require specialized equipment, lyophilized kits and simpler real-time PCR platforms are increasingly designed for robustness and ease of use, making them more accessible for low-resource settings, especially with appropriate training and infrastructure for nucleic acid extraction. The stability of lyophilized reagents significantly eases logistical burdens.
Lead Time, Warranty, and Customer Support
Ensuring operational continuity and trust in diagnostic solutions extends beyond product performance to robust logistics and support services.
Lead Time and Fulfillment
Typical lead times for standard respiratory pathogen panel kits range from 2-4 weeks, depending on inventory levels and order volume. For high-volume or customized orders, specific timelines are discussed and agreed upon during the quotation phase. We maintain robust supply chain management to minimize delays, ensuring timely delivery even during periods of high demand. Expedited shipping options are available upon request, with associated costs.
Warranty Commitments
All our diagnostic kits, including the Cowingene Mycoplasma Pneumoniae Detection Kit (Lyophilized), are provided with a warranty that guarantees performance according to the specifications outlined in the product's Instruction for Use (IFU) until the stated expiry date, provided they are stored and handled correctly. This warranty covers manufacturing defects and performance failures under normal operating conditions. Detailed warranty terms and conditions are available upon request and accompany each product shipment.
Customer Support and After-Sales Service
We are committed to providing exceptional customer support. Our technical support team, comprised of experienced molecular biologists and application specialists, is available to assist with assay setup, troubleshooting, data interpretation, and training. Support is accessible via dedicated phone lines, email, and online portals. We also offer on-site training and technical visits for complex installations or large-scale deployments, ensuring our partners achieve optimal performance from our diagnostic solutions. Our commitment to ISO 9001 quality management principles underpins our service excellence.
References
- Public Health England. (2020). COVID-19 PCR testing: guidance for laboratories. Retrieved from [Insert relevant PHE or CDC link for PCR guidance if available, otherwise general reference]
- European Centre for Disease Prevention and Control (ECDC). (2021). Laboratory testing for SARS-CoV-2 in the EU/EEA and the UK. Retrieved from [Insert relevant ECDC link if available]
- Clinical and Laboratory Standards Institute (CLSI). (2022). Molecular Diagnostic Methods for Infectious Diseases. Wayne, PA: Clinical and Laboratory Standards Institute.
- World Health Organization (WHO). (2023). Laboratory testing for respiratory pathogens. Retrieved from [Insert relevant WHO guideline link if available]
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







