Nov . 04, 2025 13:42 Kembali ke daftar
Detection Chlamydia trachomatis PCR Kit - Fast, Accurate
Detection Chlamydia Trachomatis is a key solution in the biotechnology industry, specifically within in vitro diagnosis and Molecular Diagnostics. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
Daftar isi
- Detection Chlamydia Trachomatis Overview
- Benefits & Use Cases of Detection Chlamydia Trachomatis in Molecular Diagnostics
- Biaya, Pemeliharaan & Pengalaman Pengguna
- Sustainability & Market Trends in biotechnology
- Conclusion on Detection Chlamydia Trachomatis from Taizhou Cowingene Biotech Co.,Ltd.
Detection Chlamydia Trachomatis Overview
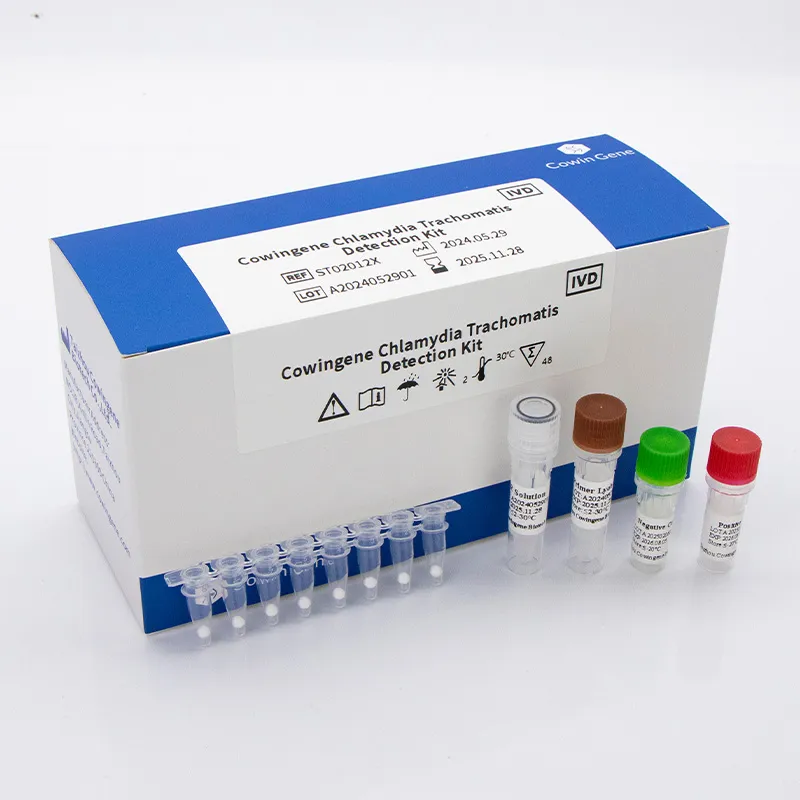
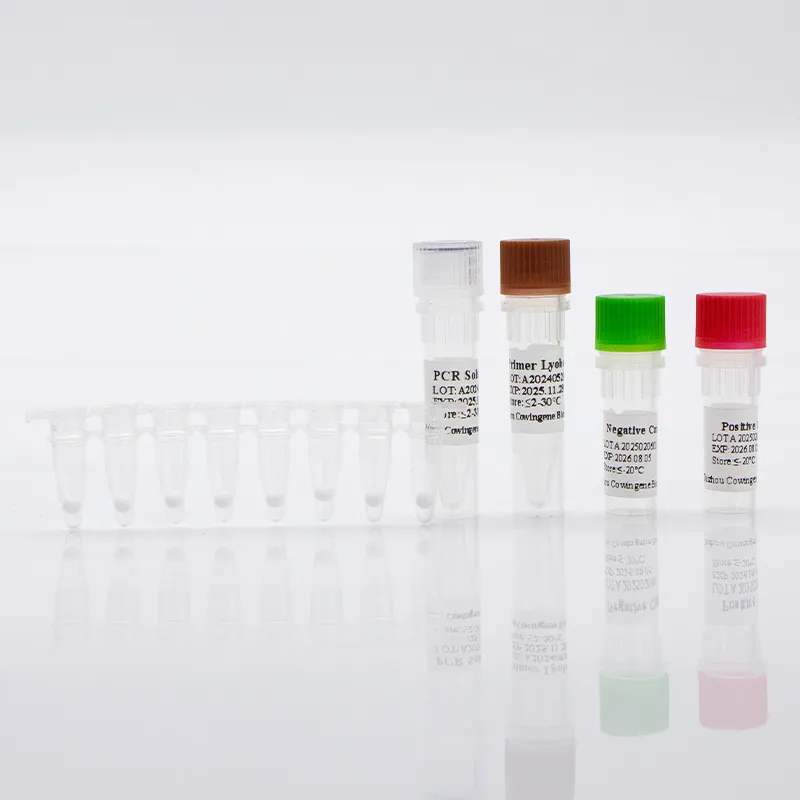
In Molecular Diagnostics, Detection Chlamydia Trachomatis typically centers on real-time PCR workflows designed to sensitively and specifically identify C. trachomatis DNA from clinical specimens such as first-catch urine or urogenital swabs. Taizhou Cowingene Biotech Co.,Ltd. provides a robust qPCR-based solution engineered for laboratory efficiency—supporting standardized extraction, internal process controls, and clear positive/negative interpretation curves. The assay aligns with established .trachomatis pcr practices and is compatible with mainstream thermocyclers used for pcr c trachomatis testing in professional labs. Technically, the kit targets conserved C. trachomatis genomic regions and integrates an internal control to monitor extraction quality and PCR inhibition, helping reduce repeat rates. Typical workflows fit seamlessly into existing nucleic acid testing pipelines and LIMS environments, enabling high-throughput screening and surveillance. Backed by Cowingene’s quality system and batch-to-batch consistency, the solution offers dependable analytical performance and operational reliability for laboratories seeking scalable, evidence-driven Detection Chlamydia Trachomatis capability.
Benefits & Use Cases of Detection Chlamydia Trachomatis in Molecular Diagnostics
High-impact applications include hospital molecular labs, public health programs, sexual health clinics, reference laboratories, and contract testing organizations. The Cowingene platform supports both routine diagnosis and population screening, providing rapid turnaround with closed-tube real-time PCR to minimize contamination risk. Key benefits include streamlined sample-to-answer workflow, clear result calling, and compatibility with automated extraction systems for scalable throughput. For B2B teams, the combination of reliable reagents, concise protocols, and assay controls translates into consistent performance across sites and operators. Competitive advantages span operational simplicity, strong reproducibility, and flexible deployment across diverse qPCR instruments. Taizhou Cowingene Biotech Co.,Ltd.’s domain expertise in Molecular Diagnostics—covering assay design, quality manufacturing, and technical documentation—helps labs accelerate validation and staff onboarding. Whether you are integrating C. trachomatis PCR into a multipathogen STI panel or running standalone screening, the Detection Chlamydia Trachomatis kit is positioned to support robust, data-driven decision making in clinical and research environments.
Biaya, Pemeliharaan & Pengalaman Pengguna
Total cost of ownership in STI testing hinges on reagent stability, hands-on time, repeat rates, and instrument utilization. Cowingene’s Detection Chlamydia Trachomatis kit is built to reduce hidden costs: concise protocols shorten training time, integrated internal controls curb invalids, and consistent batch quality stabilizes monthly consumable planning. The result is improved ROI through fewer repeats and predictable throughput. Maintenance requirements are minimal—no specialized service contracts for the kit itself—while documentation and technical support help laboratories streamline validation and ongoing QC. Feedback from in vitro diagnosis customers highlights faster turnaround, straightforward result interpretation, and dependable performance across specimen types when following validated workflows. For networked labs, the kit’s compatibility with common extraction chemistries and qPCR platforms simplifies procurement and multi-site standardization, supporting enterprise-wide efficiency and cost control.
Sustainability & Market Trends in biotechnology
The STI diagnostics market is expanding, driven by increased screening initiatives, automation, and the shift toward integrated molecular workflows. Buyers now evaluate partners on sustainability and supply-chain resilience alongside performance. Taizhou Cowingene Biotech Co.,Ltd. advances responsible manufacturing through rigorous quality systems, efficient packaging, and documentation that supports regulatory compliance where required by local markets. As laboratories emphasize greener operations—reduced plastic use, consolidated shipments, and optimized batch sizes—Cowingene aligns with procurement goals that balance environmental impact and operational continuity. Looking forward, trends such as syndromic panels, high-throughput extraction, and digital connectivity (LIMS integration, QC analytics) will continue to shape molecular testing. Cowingene’s roadmap anticipates these needs, focusing on assay robustness, platform compatibility, and professional support that enable sustainable scaling of Detection Chlamydia Trachomatis and broader STI portfolios.
Conclusion on Detection Chlamydia Trachomatis from Taizhou Cowingene Biotech Co.,Ltd.
For B2B decision makers, Detection Chlamydia Trachomatis is an essential molecular tool that underpins reliable STI screening, operational scalability, and data integrity. Taizhou Cowingene Biotech Co.,Ltd. delivers a high-performance qPCR solution that integrates seamlessly with established workflows, supports standardized QC, and helps reduce total testing costs. With strong technical support and a quality-driven manufacturing approach, Cowingene is a trusted partner for laboratories upgrading or expanding C. trachomatis PCR capabilities. Contact us: email: info@cowingene.com — Visit our website: https://www.cowingene.com
Terkait PRODUK
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
BeritaNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
BeritaNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
BeritaNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
BeritaNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
BeritaNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
BeritaNov.04,2025



























