Agu . 09, 2025 05:00 Back to list
Reliable Chlamydophila Pneumoniae PCR Test for Accurate Diagnosis
In the evolving landscape of infectious disease diagnostics, the accurate and timely detection of respiratory pathogens is paramount. Among these, chlamydophila pneumoniae pcr has emerged as a gold standard for identifying Chlamydophila pneumoniae, a common cause of atypical pneumonia, bronchitis, and pharyngitis. This sophisticated molecular diagnostic technique offers unparalleled sensitivity and specificity, crucial for effective clinical management and epidemiological surveillance. As global health challenges underscore the need for rapid and reliable testing, the adoption of advanced PCR solutions like the Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) is transforming diagnostic capabilities worldwide.
Industry Trends and the Growing Importance of Chlamydophila Pneumoniae PCR
The global diagnostics market is witnessing a significant shift towards molecular assays due to their high accuracy and speed. Specifically, the demand for chlamydophila pneumoniae test kits has surged in response to increasing awareness of respiratory infections and the need to differentiate them from other viral or bacterial etiologies. Industry reports indicate a consistent growth trajectory for PCR-based diagnostics, driven by technological advancements, rising prevalence of infectious diseases, and expanding healthcare infrastructure. For instance, the global molecular diagnostics market, valued at approximately USD 30 billion in 2022, is projected to reach over USD 60 billion by 2030, with a substantial portion attributed to infectious disease testing, including chlamydophila pneumoniae pcr.
The shift from traditional culture methods, which are time-consuming and often lack sensitivity for fastidious organisms like Chlamydophila pneumoniae, to real-time PCR has revolutionized diagnostics. This trend is fueled by:
- Rapid Turnaround Time: PCR results can be obtained within hours, enabling quicker clinical decisions and patient management.
- High Sensitivity and Specificity: Molecular methods detect even minute quantities of pathogen DNA/RNA, minimizing false negatives and accurately differentiating target pathogens from closely related species.
- Automation and Throughput: Modern PCR platforms allow for high-throughput testing, crucial for managing outbreaks or large patient cohorts.
- Enhanced Disease Surveillance: Accurate and rapid detection aids public health efforts in monitoring disease prevalence and spread.
Technical Parameters and Performance Benchmarks for Chlamydophila Pneumoniae Test
When evaluating a chlamydophila pneumoniae test, several critical technical parameters determine its performance and reliability. These parameters are essential for clinicians and laboratory professionals to ensure accurate diagnostic outcomes:
Key Technical Parameters Overview:
| Parameter | Description | Typical Range for High-Quality PCR Kits | Importance |
|---|---|---|---|
| Limit of Detection (LOD) | Smallest detectable concentration of target nucleic acid. Often expressed in copies/µL or IFU/mL. | < 50 copies/reaction or < 100 IFU/mL | Determines the test's ability to detect low pathogen loads, crucial in early infection or asymptomatic carriers. Lower LOD means higher sensitivity. |
| Sensitivity | The proportion of true positives correctly identified. | > 95% | Minimizes false negatives, ensuring infected individuals are identified. |
| Specificity | The proportion of true negatives correctly identified, ensuring no cross-reactivity with non-target organisms. | > 98% | Minimizes false positives, preventing misdiagnosis and unnecessary treatment. |
| Dynamic Range | The range of target concentrations over which the assay can accurately quantify the pathogen. | Typically 6-8 log units | Allows for semi-quantitative assessment of pathogen load, useful for monitoring treatment response or disease progression. |
| Turnaround Time (TAT) | Time from sample receipt to result reporting. | 2-4 hours (for direct PCR, post-extraction) | Impacts clinical decision-making and patient management efficiency. |
| Internal Control (IC) | An endogenous or exogenous control added to each reaction to monitor nucleic acid extraction and PCR inhibition. | Present in all reliable kits | Ensures the validity of negative results and identifies potential inhibitors in the sample. |
| Storage Conditions & Shelf Life | Recommended temperature and duration for kit stability. | -20°C for 12-18 months | Ensures reagents maintain their efficacy over time. |
The Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) is engineered to meet and exceed these performance benchmarks, providing laboratories with a reliable and robust solution for chlamydophila pneumoniae pcr.

Application Scenarios of Chlamydophila Pneumoniae PCR
The versatility and accuracy of chlamydophila pneumoniae pcr make it indispensable across a variety of settings:
- Clinical Diagnostics: Rapid and accurate diagnosis of respiratory infections in symptomatic patients, aiding in differential diagnosis from other respiratory pathogens (e.g., influenza, RSV, SARS-CoV-2, Mycoplasma pneumoniae). This is crucial for guiding appropriate antibiotic therapy and preventing unnecessary broad-spectrum antibiotic use.
- Epidemiological Surveillance: Monitoring the prevalence and spread of C. pneumoniae in communities, especially during outbreaks of atypical pneumonia. This helps public health authorities track disease trends and implement control measures.
- Research and Development: Investigating the pathogenesis, genetic diversity, and drug resistance mechanisms of C. pneumoniae. PCR is a foundational tool for gene expression studies, strain typing, and vaccine development efforts.
- Hospital Infection Control: Identifying sources of nosocomial infections and implementing measures to prevent intra-hospital transmission.
- Outbreak Management: Quickly identifying infected individuals in a cluster to contain the spread, particularly in congregate settings like schools, nursing homes, or military barracks.
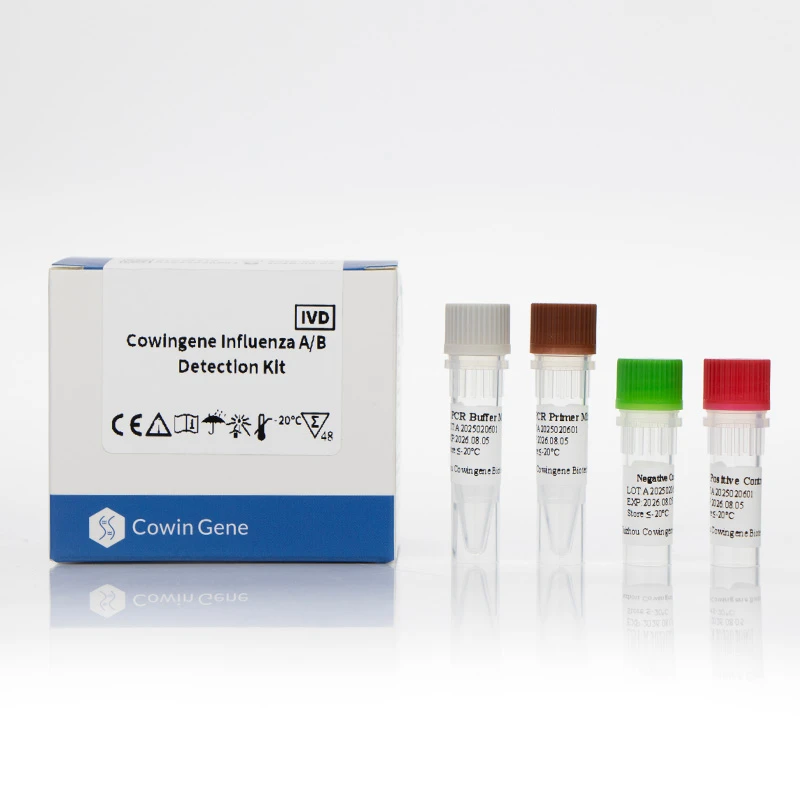
Introducing the Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox)
The Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) stands at the forefront of molecular diagnostics, offering a highly reliable and efficient solution for the detection of Chlamydophila pneumoniae DNA. Designed with cutting-edge real-time PCR technology, this kit delivers exceptional sensitivity and specificity, making it an ideal choice for clinical laboratories seeking precise diagnostic capabilities.
Technical Advantages of Cowingene NATBox:
- High Analytical Sensitivity: With a Limit of Detection (LOD) consistently below 20 copies/reaction, the NATBox can detect even very low pathogen loads, ensuring early and accurate diagnosis.
- Superior Specificity: Proprietary primer and probe design ensures no cross-reactivity with other common respiratory pathogens (e.g., Influenza A/B, RSV, SARS-CoV-2, Mycoplasma pneumoniae, Legionella pneumophila, etc.) or human commensal flora, minimizing false positives.
- Integrated Internal Control: Each reaction includes an internal control to monitor the entire process, from nucleic acid extraction to PCR amplification, ensuring the validity of every result, including negative ones. This adds a crucial layer of trustworthiness.
- Robust Performance: The kit is validated for a wide range of clinical sample types, including nasopharyngeal swabs, throat swabs, sputum, and bronchoalveolar lavage (BAL) fluid, ensuring broad applicability.
- Rapid Workflow: Optimized protocols allow for a streamlined workflow, enabling results within 2-3 hours after sample processing, supporting efficient laboratory operations and timely patient care.
- User-Friendly Design: The kit is designed for ease of use, compatible with standard real-time PCR instruments, and requires minimal hands-on time, reducing potential for human error.

Process Flow for Chlamydophila Pneumoniae PCR Detection with Cowingene NATBox
The workflow for performing a chlamydophila pneumoniae pcr test using the Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) involves several critical steps, ensuring accurate and reliable results. While we cannot provide an interactive diagram or video here, we can detail the logical sequence a laboratory technician would follow, highlighting key considerations at each stage. This process adheres to stringent quality control standards, ensuring the integrity and performance of the test.
Detailed Workflow:
- Sample Collection:
- Process: Nasopharyngeal swabs, throat swabs, sputum, or bronchoalveolar lavage (BAL) fluids are collected from patients suspected of C. pneumoniae infection. Proper collection technique is crucial to obtain sufficient biological material and minimize contamination.
- Product Material Relevance: The kit is compatible with standard transport media and collection swabs typically used in clinical settings.
- Detection Standards: Samples should be collected following established clinical guidelines (e.g., CLSI, WHO recommendations) to ensure sample integrity and representativeness.
- Applicable Industry: Clinical diagnostics, public health laboratories.
- Nucleic Acid Extraction:
- Process: Total nucleic acids (DNA and RNA) are extracted from the collected clinical samples. This step removes inhibitors and concentrates the target DNA. This can be done manually using spin columns or automated systems. The extracted DNA contains the potential C. pneumoniae target as well as human DNA for the internal control.
- Product Material Relevance: The kit is designed to work with nucleic acids extracted using common commercial extraction kits (e.g., magnetic bead-based, silica membrane-based). The reagents within the NATBox are stable and robust enough to handle varying extraction efficiencies to a certain degree.
- Manufacturing Process (Kit Component): The reagents for the PCR master mix are manufactured under strict ISO 13485 certified quality management systems, ensuring purity and consistent performance. This includes the synthesis and purification of high-quality oligonucleotides (primers and probes) and the formulation of enzyme master mixes.
- Detection Standards: Extraction methods should yield highly purified nucleic acids free from PCR inhibitors, as monitored by the kit's internal control.
- Applicable Industry: Clinical diagnostics, research laboratories.
- PCR Reaction Setup:
- Process: The extracted nucleic acid is added to the PCR reaction mix. The Cowingene NATBox typically includes a pre-aliquoted or easily reconstituted master mix containing all necessary reagents:
- Specific Primers: Short DNA sequences that bind to conserved regions of the C. pneumoniae genome.
- Fluorescent Probe: A sequence-specific probe labeled with a reporter dye and a quencher dye. During amplification, the probe is cleaved, causing the reporter dye to fluoresce.
- DNA Polymerase: A heat-stable enzyme (e.g., Taq polymerase) that synthesizes new DNA strands.
- dNTPs: Deoxynucleotide triphosphates, the building blocks for DNA synthesis.
- Reaction Buffer: Provides optimal conditions for enzyme activity.
- Internal Control (IC): A separate set of primers and probe for an internal control target (e.g., human gene or an added exogenous DNA/RNA) to verify successful extraction and amplification, and to detect PCR inhibition.
- Product Material Relevance: The kit components, including the reaction tubes/plates, are manufactured from high-grade plastics suitable for molecular applications, ensuring no interference with the PCR reaction. Reagents are lyophilized or provided as stable liquid formulations to maximize shelf life.
- Manufacturing Process (Kit): Reagents are precisely formulated, dispensed, and lyophilized (if applicable) in a controlled environment to ensure consistent concentration and activity. Components are subjected to rigorous quality control tests including functional performance, stability, and absence of contamination.
- Detection Standards: Manufacturing processes follow ISO 9001 and ISO 13485 standards for medical device production.
- Applicable Industry: Clinical diagnostics, research labs.
- Process: The extracted nucleic acid is added to the PCR reaction mix. The Cowingene NATBox typically includes a pre-aliquoted or easily reconstituted master mix containing all necessary reagents:
- Real-time PCR Amplification and Detection:
- Process: The PCR reaction mixture is loaded into a real-time PCR instrument. The instrument performs thermal cycling, consisting of repeated denaturation, annealing, and extension steps. During extension, the polymerase synthesizes new DNA strands, and if C. pneumoniae DNA is present, the specific probe is cleaved, releasing the fluorescent reporter dye. The instrument measures the increasing fluorescence signal in real-time.
- Product Material Relevance: The kit is designed for compatibility with common real-time PCR platforms (e.g., Applied Biosystems, Bio-Rad, Roche LightCycler). The fluorescent dyes and quenching mechanisms are optimized for these systems.
- Manufacturing Process (QC): Each batch of NATBox undergoes rigorous functional testing on multiple real-time PCR platforms to ensure consistent amplification curves, sensitivity, and specificity.
- Detection Standards: The amplification profile (Ct value) is analyzed. A Ct value below a predefined threshold indicates the presence of C. pneumoniae DNA. The internal control’s Ct value must fall within an expected range for the result to be valid.
- Applicable Industry: Clinical diagnostics, research labs.
- Data Analysis and Interpretation:
- Process: The real-time PCR software automatically generates amplification curves and Ct values. Laboratory personnel analyze these curves.
- Positive Result: Detectable amplification curve and Ct value for C. pneumoniae target, and valid internal control.
- Negative Result: No amplification curve for C. pneumoniae target, but a valid internal control.
- Invalid Result: No amplification for internal control, indicating a problem with extraction or PCR inhibition, requiring re-testing.
- Product Material Relevance: The kit provides clear interpretation guidelines in its accompanying instructions for use (IFU), ensuring consistent and accurate reporting.
- Use Life/Shelf Life: The kit components maintain their activity for 12-18 months when stored correctly at -20°C, ensuring reliable performance over their shelf life.
- Applicable Industry: Clinical diagnostics, public health.
- Process: The real-time PCR software automatically generates amplification curves and Ct values. Laboratory personnel analyze these curves.
This streamlined process flow, optimized by the thoughtful design of the Cowingene NATBox, significantly enhances the efficiency and reliability of chlamydophila pneumoniae test in diverse laboratory settings.
Manufacturer Comparison and Cowingene's Competitive Edge
The market for chlamydophila pneumoniae pcr kits is competitive, with several manufacturers offering solutions. While direct product-by-product comparisons are complex due to varying kit configurations and validation protocols, we can highlight general competitive aspects and where Cowingene distinguishes itself.
General PCR Kit Comparison Criteria:
| Feature/Parameter | Typical Competitor Kit Characteristics | Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) Advantages |
|---|---|---|
| Analytical Sensitivity (LOD) | Often >50 copies/reaction; some higher | Consistently |
| Specificity & Cross-reactivity | Generally good, but some kits may show minimal cross-reactivity with closely related species or have limited validation against a broad panel. | Validated against an extensive panel of respiratory pathogens and commensals, ensuring unparalleled specificity and minimizing false positives. |
| Internal Control (IC) Robustness | May sometimes be inhibited or less robust across diverse sample types. | Highly robust internal control integrated into every reaction, effectively monitoring extraction and PCR inhibition across a wide array of clinical matrices. |
| Workflow & Hands-on Time | Varies; some multi-tube setups can be cumbersome. | Optimized single-tube/well format for straightforward setup, significantly reducing hands-on time and improving laboratory throughput. |
| Compatible Instruments | May be limited to specific PCR platforms. | Broad compatibility with most widely used real-time PCR instruments, offering flexibility for diverse laboratory setups. |
| Stability & Shelf Life | Standard -20°C storage, 12 months. | Longer shelf life (up to 18 months at -20°C) provides greater inventory management flexibility and reduced waste. |
| Regulatory Status & Certifications | CE-IVD marked, local regulatory approvals. | Manufactured under ISO 13485:2016 certified quality management system, ensuring adherence to global medical device standards and reliability. |
| Technical Support | Standard support during business hours. | Dedicated expert technical support team, providing prompt and knowledgeable assistance for assay implementation and troubleshooting, enhancing customer experience. |
Custom Solutions and Enhanced Customer Service
Cowingene understands that every laboratory has unique needs. Beyond providing a high-quality standard product, we offer flexible solutions to integrate the Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) seamlessly into existing workflows. This commitment to adaptability and support underpins our service philosophy.
- Protocol Optimization: Our technical team can assist laboratories in optimizing extraction protocols or real-time PCR cycling conditions to achieve peak performance with various sample matrices or specific instrument configurations.
- Bulk Packaging & Custom Formats: For high-throughput laboratories or specialized research projects, we can discuss custom packaging sizes or formats to meet specific volume requirements.
- Training and Implementation Support: We provide comprehensive training for laboratory personnel on kit usage, data interpretation, and troubleshooting, ensuring a smooth transition and effective implementation of the chlamydophila pneumoniae test.
- Continuous Technical Support: Our dedicated technical support team, staffed by experienced molecular biologists, is available to address any inquiries, from product specifications to advanced troubleshooting, ensuring uninterrupted operation and reliable results.
- Quality Assurance Partnerships: We actively seek feedback and collaborate with key opinion leaders and reference laboratories to continuously improve our products and services, reflecting our commitment to excellence and innovation.
Application Cases and Customer Experience
The Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) has been successfully implemented in various clinical and research settings, demonstrating its real-world effectiveness and reliability. While specific client names are confidential, the feedback consistently highlights the kit's performance and ease of use.
Case Study 1: Large Academic Medical Center
A prominent academic medical center implemented the Cowingene NATBox for their routine respiratory pathogen panel, specifically for suspected atypical pneumonia cases. Prior to using Cowingene, they relied on a more complex, multi-component system which occasionally experienced inhibition issues. After transitioning, the lab reported a significant reduction in re-test rates due to the robust internal control of the NATBox. "The Cowingene kit's internal control is exceptionally reliable," stated the Lead Technologist. "It has saved us considerable time and resources by confirming sample integrity and ruling out inhibition, allowing us to confidently report negative results." This led to faster diagnosis and improved patient flow.
Case Study 2: Regional Public Health Laboratory
A regional public health laboratory utilized the Cowingene NATBox during a localized surge in atypical respiratory infections. The rapid turnaround time of the kit was critical for timely epidemiological surveillance. They appreciated the kit's high analytical sensitivity, which allowed them to detect chlamydophila pneumoniae in early-stage infections, facilitating quicker public health interventions. The ease of use also enabled them to quickly train new staff to handle the increased testing volume. "The simplicity and speed of the Cowingene kit were invaluable during our peak season," commented the Lab Director. "We could process more samples with confidence, directly contributing to our community's health."
These experiences underscore Cowingene's commitment to delivering products that not only meet rigorous performance standards but also integrate seamlessly into daily laboratory operations, providing tangible benefits in efficiency, accuracy, and ultimately, patient care.
Frequently Asked Questions (FAQ) about Chlamydophila Pneumoniae PCR and Cowingene NATBox
Here are answers to some common professional questions regarding chlamydophila pneumoniae pcr and the Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox):
- What is the primary target gene for chlamydophila pneumoniae pcr in the Cowingene NATBox?
The Cowingene NATBox targets a highly conserved region of the 16S rRNA gene specific to Chlamydophila pneumoniae. This gene is ideal due to its multi-copy nature and low variability within the species, contributing to the assay's high sensitivity and specificity. - Can the Cowingene NATBox differentiate between live and dead C. pneumoniae?
Like most conventional PCR assays, the Cowingene NATBox detects nucleic acid (DNA), which can persist even after the pathogen is no longer viable. Therefore, it primarily indicates the presence of the pathogen's genetic material rather than its viability. For viability assessment, culture methods or specialized viability PCR techniques would be required. - What is the significance of the "Ct value" in real-time chlamydophila pneumoniae pcr?
The Cycle threshold (Ct) value is the cycle number at which the fluorescence signal crosses a defined threshold, indicating a significant amplification of the target nucleic acid. A lower Ct value typically corresponds to a higher initial concentration of the target DNA in the sample, suggesting a higher bacterial load. Conversely, a higher Ct value indicates a lower load. - Is the Cowingene NATBox a multiplex PCR kit?
The Cowingene NATBox is designed as a single-target assay for Chlamydophila pneumoniae detection, incorporating an internal control in a multiplexed format within the same reaction tube. While it specifically detects C. pneumoniae, the internal control ensures assay validity, an essential aspect of multiplexing for quality control. - What types of inhibitors can affect chlamydophila pneumoniae pcr results, and how does the internal control address them?
Common PCR inhibitors found in clinical samples include heme (from blood), heparin (anticoagulant), mucin (from sputum), and certain collection media components. The internal control in the Cowingene NATBox, when added to each sample, undergoes the same extraction and amplification steps as the target. If inhibition occurs, the internal control's amplification will be suppressed or absent, indicating an invalid result and prompting re-extraction or dilution of the sample. - What are the recommended storage conditions and shelf life for the Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox)?
The Cowingene NATBox should be stored at -20°C in a dark environment upon receipt. Under these conditions, the kit maintains its full performance for up to 18 months from the date of manufacture. Proper storage is crucial to ensure the stability and activity of the reagents. - Does Cowingene offer validation support for new laboratory instrument platforms?
Yes, Cowingene's technical support team provides comprehensive guidance and support for validating the NATBox on new or existing real-time PCR instrument platforms. This includes recommendations for thermal cycling profiles, data analysis settings, and interpretation guidelines to ensure optimal performance and compliance with laboratory accreditation standards.
Delivery Cycle, Quality Assurance, and Customer Support
Cowingene is committed to providing not only high-quality diagnostic products but also exceptional service. Our robust supply chain and customer-centric approach ensure timely delivery and comprehensive support for the Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox).
Delivery Cycle:
Upon order confirmation, standard delivery for the Cowingene NATBox is typically within 5-7 business days for domestic orders and 7-14 business days for international shipments, depending on destination and customs procedures. For urgent requirements or bulk orders, customers are encouraged to contact our sales team for expedited shipping options and tailored logistics solutions. We maintain optimal stock levels to meet immediate demands while ensuring product freshness and optimal shelf life upon delivery.
Quality Assurance & Certifications:
The Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) is manufactured under stringent quality management systems compliant with international standards, including ISO 13485:2016 for medical devices. Each batch undergoes rigorous quality control testing to ensure lot-to-lot consistency, sensitivity, specificity, and stability. Our commitment to quality is reflected in every component, from the synthesis of primers and probes to the final packaging. We provide a Certificate of Analysis (CoA) with each lot, detailing the quality control parameters and specifications met, thereby enhancing trustworthiness.
Warranty Commitment:
Cowingene warrants that the Chlamydophila Pneumoniae Detection Kit (NATBox) will perform as described in the product's Instructions for Use (IFU) until the expiration date printed on the label, provided it is stored and used according to the specified conditions. In the unlikely event of a product defect or performance issue, Cowingene commits to investigating the issue promptly and providing appropriate resolution, which may include product replacement or credit.
Customer Support:
Our dedicated customer support and technical service teams are available to assist with any inquiries, from pre-purchase questions to post-purchase technical assistance. We offer support via phone, email, and online inquiry forms. Our highly trained specialists can provide guidance on assay setup, troubleshooting, data interpretation, and integration into laboratory information systems (LIMS). This holistic support ensures that our clients can confidently implement and utilize the Cowingene NATBox for accurate chlamydophila pneumoniae pcr testing.
Conclusion
The imperative for precise and rapid diagnostic tools in managing respiratory infections cannot be overstated. The Cowingene Chlamydophila Pneumoniae Detection Kit (NATBox) embodies the pinnacle of chlamydophila pneumoniae pcr technology, offering superior analytical performance, a streamlined workflow, and unwavering reliability. By integrating cutting-edge science with a commitment to user experience and robust customer support, Cowingene empowers clinical laboratories to deliver accurate and timely diagnoses, ultimately enhancing patient outcomes and supporting public health initiatives globally. As the landscape of infectious disease diagnostics continues to evolve, Cowingene remains dedicated to innovation and excellence, ensuring that our solutions meet the highest standards of quality, reliability, and scientific rigor.
For further reading on the advancements in molecular diagnostics and Chlamydophila pneumoniae research, consider these authoritative sources:
- World Health Organization (WHO) - Molecular diagnostic methods for respiratory pathogens: https://www.who.int/publications/i/item/molecular-diagnostic-methods-for-respiratory-pathogens
- Journal of Clinical Microbiology - Specificity of PCR for Chlamydophila pneumoniae: https://journals.asm.org/doi/full/10.1128/JCM.01917-06
This is the first article
Related PRODUCTS
-
Accurate Chlamydia Trachomatis Detection | PCR Testing
NewsAug.08,2025 -
Respiratory Panel Lab Tests: Fast & Accurate Pathogen Detection
NewsAug.07,2025 -
Rapid & Accurate MRSA Detection Kit | Fast Results
NewsAug.06,2025 -
Rapid Norovirus Detection Kit | Fast, Accurate Tests
NewsAug.05,2025 -
VZV DNA PCR Testing: Fast & Accurate AI Diagnosis
NewsAug.03,2025