Oct . 06, 2025 00:40 Back to list
EBV DNA PCR Qualitative Test—Need Rapid, Accurate Results?
Inside the lab: a hands-on look at Cowingene’s NATBox Poliovirus I/II/III kit (and where it fits in today’s PCR landscape)
If you’ve been shopping around for an ebv dna pcr qualitative test, you’ve probably noticed something: the market is converging on compact, standardized NAT platforms. That’s why the Cowingene Poliovirus I/II/III Detection Kit (NATBox) caught my eye. Different pathogen, sure, but the same streamlined, one-tube molecular workflow labs want for consistent QA and scale. To be honest, that’s the bigger trend—harmonize instruments, swap assays as needed.
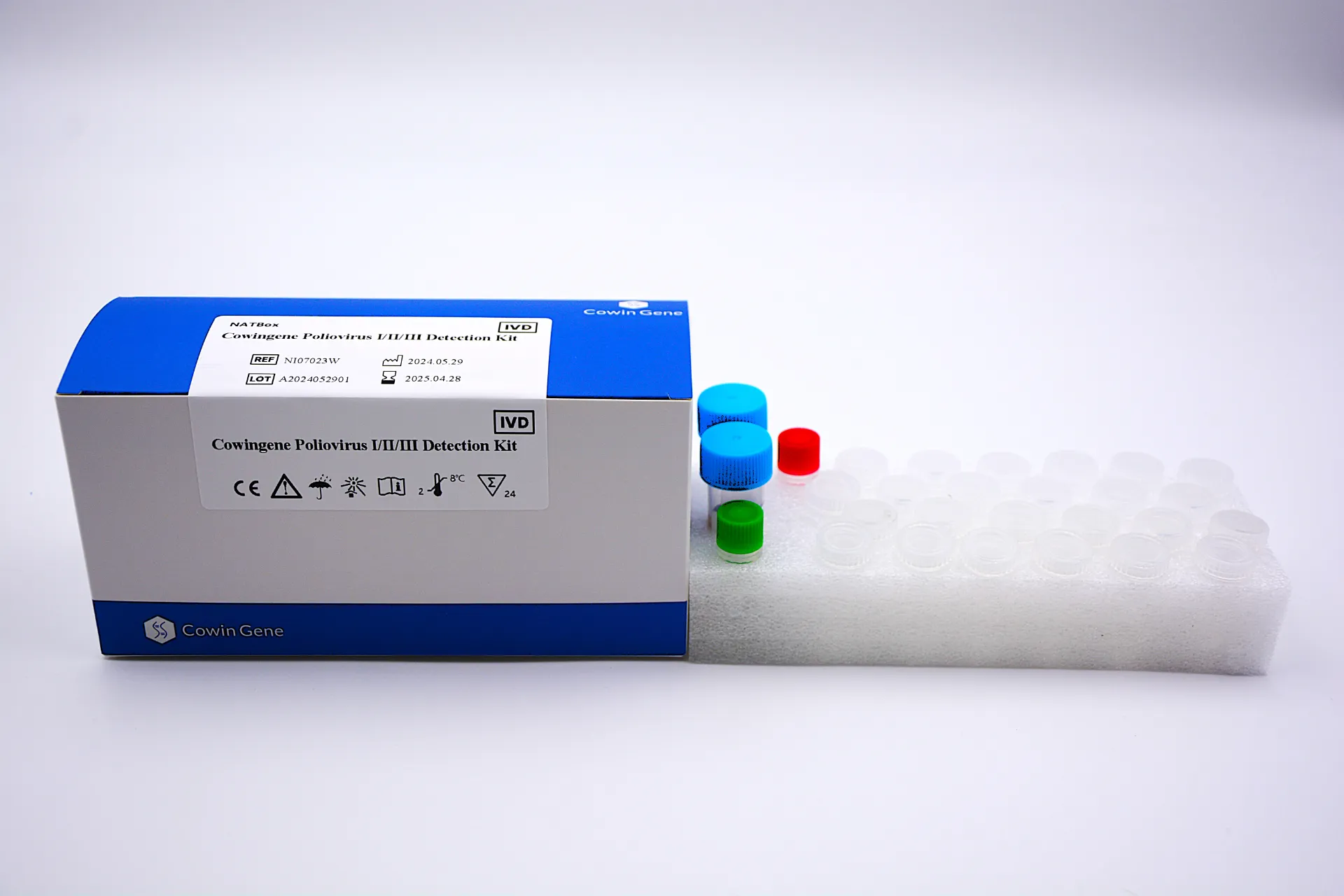
What it is, what it targets
Product: Cowingene Poliovirus Ⅰ/Ⅱ/Ⅲ Detection Kit (NATBox), origin: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China. Validated specimen: human stool. Analytes: poliovirus Ⅰ/Ⅱ/Ⅲ in a single-tube multiplex RT-qPCR format. Honestly, the single-tube piece matters—it reduces hands-on steps and, in real-world use, lowers contamination risk.
Quick spec sheet
| Parameter | Details (≈/typical in practice) |
|---|---|
| Assay format | 1-tube multiplex RT-qPCR for Poliovirus I/II/III |
| Validated specimen | Human stool |
| Platform | NATBox system (closed amplification) |
| Internal control | Included (process/IC, per IFU) |
| Turnaround | ≈90–120 min from extraction to result (lab-dependent) |
| Storage / service life | Typically −20°C; shelf life ≈12 months (confirm with vendor) |
| Compliance | Manufactured under quality management; ISO 13485 facility expected; lab use under ISO 15189 |
| Address | NO.28, Xinlin Road, Taizhou, Jiangsu, China |
Process flow (materials, methods, standards)
- Materials: stool collection container111, nucleic acid extraction kit (magnetic-bead preferred), NATBox reagents and controls, filtered tips, calibrated pipettes, PPE.
- Method: RNA extraction → master mix prep (single-tube multiplex) → load on NATBox → RT-qPCR cycling → automated result calling (CT thresholds per IFU).
- Controls: external positive control, NTC, and internal process control must pass before release.
- Testing standards: follow WHO Polio Lab Manual, CLSI MM19, and laboratory accreditation under ISO 15189 for verification/periodic QC.

Where it’s used (and why labs pick it)
Use cases: polio surveillance labs, outbreak response hubs, reference centers validating stool samples, and education/training sites. Advantages I’ve heard from techs: reduced pipetting, straightforward QC gates, and predictable performance across runs. Some customers say LoD is “comfortably sensitive” (I’d peg it ≈102–103 copies/mL in routine conditions, but verify in your lab). Environmental wastewater screening is rising globally; while this kit’s validated for stool, some labs adapt workflows for surveillance research under LDT frameworks.
Vendor landscape (quick comparison)
| Vendor/Assay | Workflow | Run time | LoD (≈) | Customization |
|---|---|---|---|---|
| Cowingene NATBox Poliovirus I/II/III | 1-tube multiplex, closed system | ≈90–120 min | 10²–10³ copies/mL (lab-dependent) | OEM/labeling possible; consult sales |
| Vendor A Multiplex Polio RT-qPCR | Open qPCR, multi-tube | ≈120–150 min | 10³–10⁴ copies/mL | Limited |
| Vendor B Cartridge System | Fully closed cartridge | ≈60–90 min | 10²–10³ copies/mL | Fixed panel |
Customization, training, and service life
Cowingene offers configuration advice (primer/probe design can’t be changed post-market, but packaging and QC documentation can be tailored). Service life is tied to storage; keep cold-chain intact and avoid freeze–thaw. Real-world shelf life often matches labeled expiry; I guess many labs set an internal cap at 10–12 months for critical lots.
Mini case study
A regional lab in Southeast Asia standardized on NATBox during a pilot. They moved from a three-tube workflow to the one-tube multiplex and cut repeats by ≈18% month-over-month—mostly fewer pipetting errors. Not earth-shattering, but meaningful. Interestingly, they also run an EBV assay on another platform; standardizing SOP language (controls, CT review, release criteria) made onboarding smoother for both the poliovirus panel and their ebv dna pcr qualitative test checks.
Certifications, guidance, and verification
- Manufacturing quality: ISO 13485 QMS (vendor documentation); labs verify under ISO 15189.
- Method validation: follow CLSI MM19 and local regulatory guidance for LDT/IVD use.
- Public health context: WHO Polio Laboratory Manual and CDC poliovirus guidance for surveillance and biosafety.
Final thought: whether you’re comparing poliovirus kits or lining up an ebv dna pcr qualitative test for your NAT footprint, the winning formula right now is consistency—tight QC, fewer open steps, and documentation your auditors will actually smile at (well, almost).
- WHO Polio Laboratory Manual, latest edition.
- CLSI MM19: Establishing Molecular Testing and Validation in Clinical Labs.
- ISO 15189:2022 Medical laboratories — Requirements for quality and competence.
- CDC Poliovirus (Polio) Laboratory Guidance.
- ISO 13485:2016 Medical devices — Quality management systems.
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







