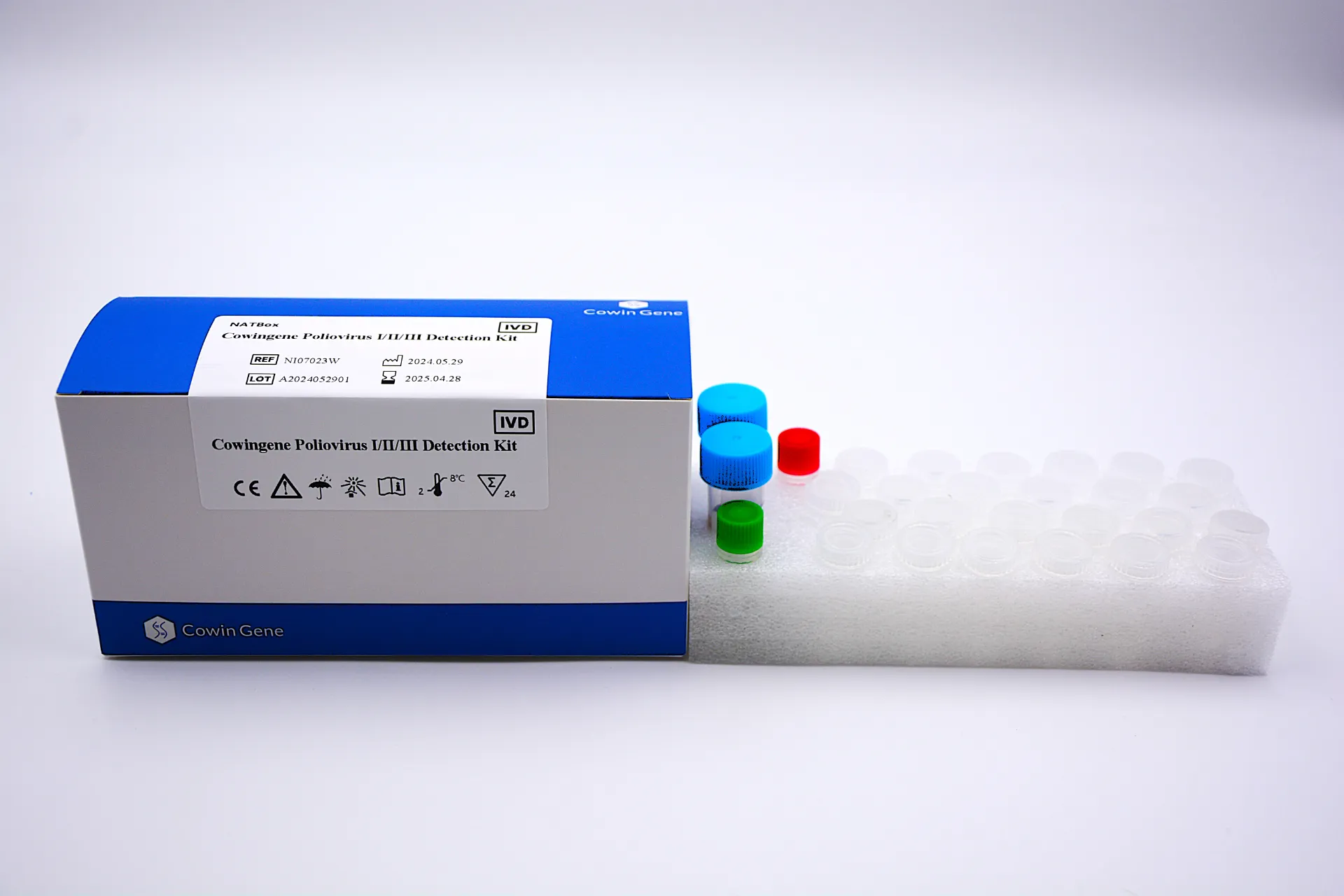
Cowingene Poliovirus Ⅰ/Ⅱ/Ⅲ Detection Kit(Lyophilized)
The Cowingene Poliovirus Ⅰ/Ⅱ/Ⅲ Detection Kit (PV Ⅰ/Ⅱ/Ⅲ) Test is an in vitro diagnostic test for the qualitative detection of RNA from Poliovirus Ⅰ/Ⅱ/Ⅲ in patient specimens. The test utilizes amplification of target RNA by the Polymerase Chain Reaction (PCR) for the detection of Poliovirus Ⅰ/Ⅱ/Ⅲ in a single analysis. The test specifically identifies Poliovirus Ⅰ/Ⅱ/Ⅲ.
Order Information
| REF | Product Name | Type | Package Size |
| NI07022X | Cowingene Poliovirus Ⅰ/Ⅱ/Ⅲ Detection Kit | Lyophilized | 48 tests/kit |
Specification
| Detection Target |
Poliovirus Ⅰ/Ⅱ/Ⅲ |
| Storage |
2-30℃ for 18 months |
| LoD |
The LoD of the kit for detecting Poliovirus Ⅰ/Ⅱ/Ⅲ is 400 copies/mL. |
| Validated Specimen |
Human stool |
| Compatible Instruments |
Open real time PCR machine with channels FAM, VIC/HEX, ROX, CY5, i.e.Roche 480, Bio-Rad CFX96,ABI7500,SLAN96, etc. |
STORAGE AND HANDLING REQUIREMENTS
A. Reagents can be stored at 2-30℃ for 18 months
B. Do not use reagents past expiration date indicated on outside of package
C. Protect from light
D. Once opened, reagent is valid for 7 days at 2-8℃
E. Once added PCR Solution, positive control and negative control are valid for 7 days at 2-8℃
Recommended Supporting Reagents
| REF | Product Name | Certification | Package Size |
| CW05021X | Cowingene DNA/RNA Extraction kit | CE | 48 tests/kit |
| CW10021Y | Cowingene Viral DNA/RNA Column | CE | 50 tests/kit |