พ.ย. . 04, 2025 13:40 กลับไปยังรายการ
Detection Chlamydia trachomatis PCR: Rapid, Accurate
Detection Chlamydia Trachomatis is a key solution in the healthcare industry, specifically within in vitro diagnosis and Molecular Diagnostics. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
สารบัญ
- Detection Chlamydia Trachomatis Overview
- Benefits & Use Cases of Detection Chlamydia Trachomatis in Molecular Diagnostics
- ต้นทุน การบำรุงรักษา และประสบการณ์ผู้ใช้
- Sustainability & Market Trends in healthcare
- Conclusion on Detection Chlamydia Trachomatis from Taizhou Cowingene Biotech Co.,Ltd.
Detection Chlamydia Trachomatis Overview
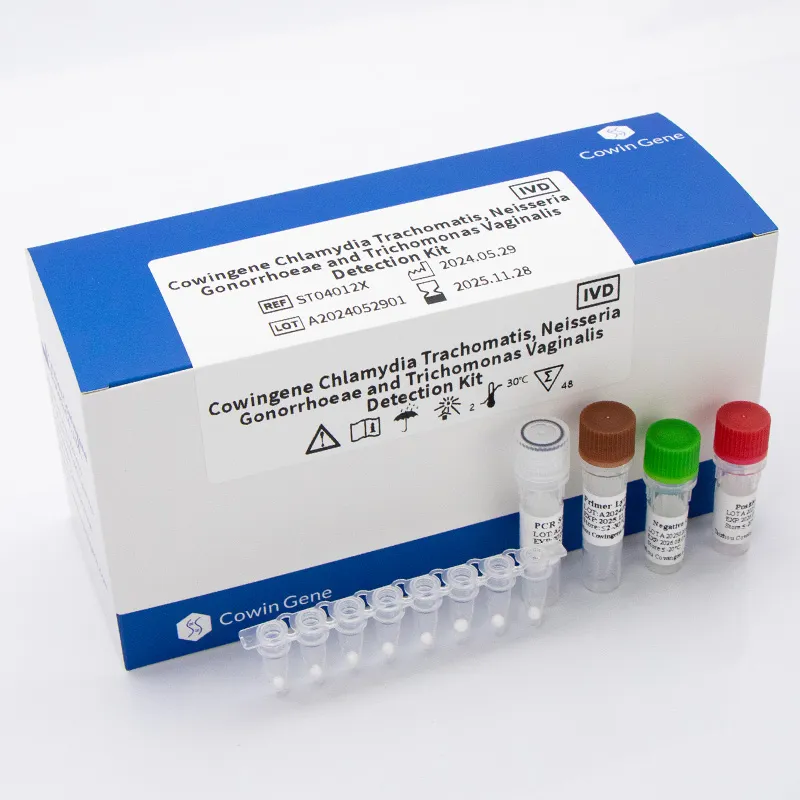
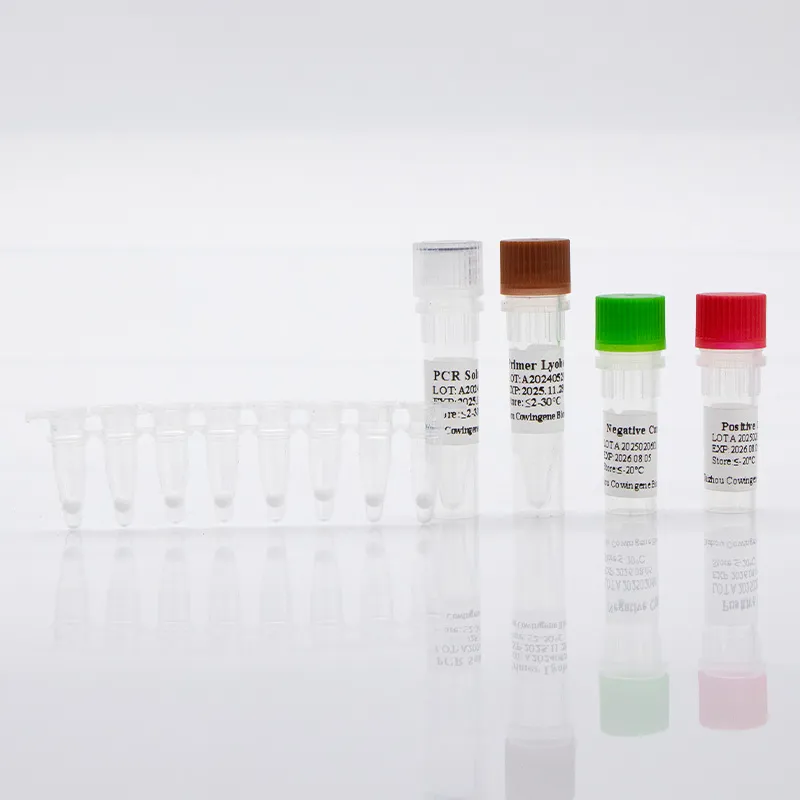
In modern STI screening pathways, Detection Chlamydia Trachomatis via nucleic acid amplification testing (NAAT) is the clinical standard for accuracy and speed. Taizhou Cowingene Biotech Co.,Ltd. provides an open-platform, real-time PCR assay designed for reliable identification of Chlamydia trachomatis DNA across common specimen types, including first-void urine and urogenital swabs. In many laboratories, C. trachomatis PCR—often referred to as trachomatis PCR or PCR C. trachomatis—enables sensitive, specific results that fit high-throughput workflows without sacrificing turnaround time.
Built for Molecular Diagnostics, the assay incorporates an internal control to monitor extraction and amplification, supports multiplexing with Neisseria gonorrhoeae targets, and is compatible with mainstream qPCR instruments. Target design typically covers conserved genomic regions linked to inclusivity across prevalent serovars, with stringent cross-reactivity screening to reduce false positives. From extraction through amplification, the workflow is streamlined to help labs meet service-level agreements while maintaining quality. With strong manufacturing controls and documented lot traceability, Taizhou Cowingene Biotech Co.,Ltd. is a reliable partner for in vitro diagnosis programs scaling from pilot sites to nationwide networks.
Benefits & Use Cases of Detection Chlamydia Trachomatis in Molecular Diagnostics
Use cases span centralized reference labs, regional hospitals, and public health screening initiatives. NAAT-based Detection Chlamydia Trachomatis supports routine screening, confirmatory testing, and co-testing with N. gonorrhoeae in symptomatic and asymptomatic populations. For high-throughput environments, open-platform compatibility simplifies integration with existing extraction systems and liquid handlers, allowing laboratories to scale volumes while keeping per-test costs predictable.
Key advantages include robust analytical sensitivity, internal control for process verification, and multiplex capability to reduce sample re-processing. The assay’s design supports consistent performance in the presence of common inhibitors found in urine and swab matrices—critical for real-world reliability. Taizhou Cowingene Biotech Co.,Ltd. brings deep assay development expertise, offering technical documentation, training, and responsive support to help teams validate and deploy C. trachomatis PCR at scale. By aligning assay features with operational needs, labs can shorten turnaround times and strengthen clinical confidence.
ต้นทุน การบำรุงรักษา และประสบการณ์ผู้ใช้
Total cost of ownership for Detection Chlamydia Trachomatis hinges on reagent stability, workflow efficiency, and minimized repeat testing. Cowingene’s kit options and pack sizes help align lot usage with sample volumes to reduce waste. Internal controls and optimized master mixes lower the risk of invalids, limiting reruns and preserving technician time. For most labs, extraction-to-result workflows can be completed within typical NAAT timelines, enabling same-day reporting while maintaining cost discipline.
Users report straightforward setup, clear IFU documentation, and smooth onboarding thanks to open-instrument compatibility. Routine maintenance is minimal: standard qPCR system care, periodic calibration, and adherence to QC schedules. The result is a favorable ROI driven by dependable performance, scalable throughput, and predictable reagent consumption. For B2B decision makers responsible for service quality and margins, these factors translate into operational resilience and improved patient pathway efficiency.
Sustainability & Market Trends in healthcare
The global STI diagnostics market continues to expand as health systems scale NAAT adoption and broaden screening access. Regulatory expectations are rising, with greater emphasis on analytical validation, risk management, and post-market surveillance. In this environment, suppliers that provide transparent quality systems and robust technical files help laboratories maintain compliance and mitigate supply-chain risk. Detection Chlamydia Trachomatis assays that are open-platform and documentation-rich are strategically advantageous for multi-site lab networks adapting to evolving standards.
Sustainability also matters. Laboratories increasingly favor vendors that reduce packaging waste, enable consolidated shipments, and support optimized batch sizes to limit cold-chain frequency where feasible. Taizhou Cowingene Biotech Co.,Ltd. positions itself as forward-thinking, with a focus on product reliability, consistent lot quality, and practical measures that can help partners shrink environmental footprints across procurement, storage, and use. This combination of technical rigor and eco-conscious operations strengthens long-term partnerships in Molecular Diagnostics.
Conclusion on Detection Chlamydia Trachomatis from Taizhou Cowingene Biotech Co.,Ltd.
For labs seeking dependable NAAT performance, Detection Chlamydia Trachomatis from Taizhou Cowingene Biotech Co.,Ltd. delivers the sensitivity, specificity, and operational efficiency required for modern screening programs. With open-platform compatibility, built-in controls, and expert technical support, Cowingene is a trusted partner for scaling C. trachomatis PCR across diverse testing settings. Contact us: info@cowingene.com. Visit our website: https://www.cowingene.com.
ที่เกี่ยวข้อง สินค้า
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
ข่าวNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
ข่าวNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
ข่าวNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
ข่าวNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
ข่าวNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
ข่าวNov.04,2025



























