Eyl . 22, 2025 10:44 Listeye geri dön
Accurate Chlamydophila Pneumoniae PCR Test | Rapid Detection
The Precision of Chlamydophila Pneumoniae PCR: A Core Diagnostic Technology
In the landscape of clinical diagnostics, the rapid and accurate identification of pathogens is paramount. One such critical area involves respiratory infections, where chlamydophila pneumoniae pcr stands out as a superior method for detecting the causative agent of atypical pneumonia and other respiratory tract infections. This advanced molecular diagnostic technique offers unparalleled sensitivity and specificity, crucial for effective patient management and public health surveillance.
Chlamydophila pneumoniae (now officially reclassified as Chlamydia pneumoniae) is a common human respiratory pathogen responsible for a significant proportion of community-acquired pneumonia, bronchitis, sinusitis, and pharyngitis. Its widespread prevalence and the potential for severe complications, particularly in immunocompromised individuals or the elderly, underscore the urgent need for reliable detection methods. Traditional diagnostic approaches, such as culture or serology, often suffer from long turnaround times, low sensitivity, or cross-reactivity issues. In contrast, PCR-based assays provide direct detection of bacterial DNA, ensuring rapid and highly accurate results.
This comprehensive overview delves into the technical intricacies, application benefits, and strategic advantages of utilizing chlamydophila pneumoniae pcr in modern B2B diagnostic settings, addressing critical industry trends, technical specifications, and real-world implementation.
Industry Trends in Molecular Diagnostics for Respiratory Pathogens
The global molecular diagnostics market is experiencing robust growth, driven by an increasing incidence of infectious diseases, advancements in diagnostic technologies, and a growing emphasis on personalized medicine. For respiratory pathogens like Chlamydophila pneumoniae, several key trends are shaping the future of testing:
- Syndromic Panels: A shift from single-target PCR to multiplex PCR panels that can simultaneously detect multiple respiratory pathogens (viruses and bacteria, including Chlamydophila pneumoniae) from a single sample. This enhances diagnostic efficiency, especially during flu season.
- Point-of-Care Testing (POCT): Development of portable, rapid PCR systems that can deliver results closer to the patient, reducing turnaround times and improving clinical decision-making. While full chlamydophila pneumoniae pcr still often requires laboratory settings, efforts towards rapid, near-patient solutions are ongoing.
- Automation and Integration: Increased demand for fully automated nucleic acid extraction and PCR platforms to minimize manual intervention, reduce human error, and increase throughput in high-volume laboratories.
- Improved Sensitivity and Specificity: Continuous innovation in primer design, probe chemistry, and real-time PCR instrumentation to achieve even higher analytical performance, particularly for challenging clinical samples.
- Digital Health Integration: Integration of diagnostic results with electronic health records (EHR) and laboratory information management systems (LIMS) for seamless data flow and enhanced epidemiological tracking.
These trends highlight a clear industry direction towards more comprehensive, faster, and more efficient diagnostic solutions, where chlamydophila pneumoniae pcr remains a foundational element for bacterial detection.
Technical Specifications and Parameters for Chlamydophila Pneumoniae PCR Kits
A robust chlamydophila pneumoniae test relies on meticulously engineered PCR kits. Key performance characteristics define their utility in clinical settings. The Cowingene Chlamydophila Pneumoniae Detection Kit (Liquid) exemplifies these advanced features.
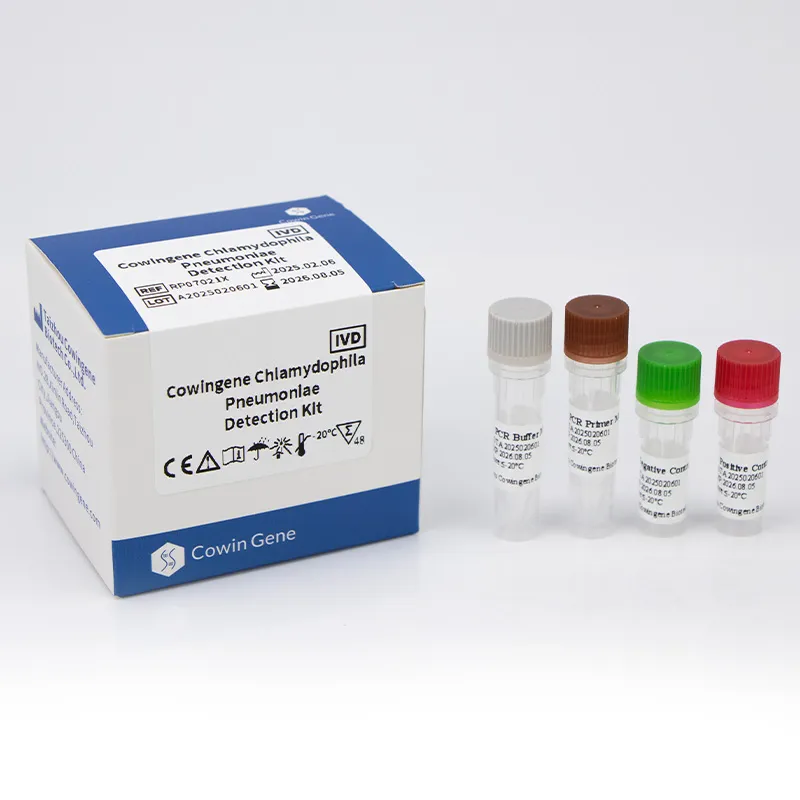
Cowingene Chlamydophila Pneumoniae Detection Kit (Liquid) - Key Specifications
| Parametre | Şartname |
|---|---|
| Target Gene | Highly conserved region of the C. pneumoniae genome (e.g., ompA, 16S rRNA) |
| Tespit Yöntemi | Real-time Polymerase Chain Reaction (qPCR) with fluorescent probes |
| Sample Type Compatibility | Nasopharyngeal swabs, oropharyngeal swabs, sputum, bronchoalveolar lavage (BAL) fluid |
| Sensitivity (Limit of Detection) | Typically < 500 copies/mL (e.g., 200 copies/mL, 95% CI) |
| Özgüllük | > 99% (no cross-reactivity with common respiratory flora or other pathogens) |
| Reaction Volume | 20-25 µL per reaction |
| Tepki Süresi | Approximately 60-90 minutes (post-extraction) |
| Raf ömrü | 18 months from manufacturing date (stored at -20°C) |
| Certifications | CE-IVD, ISO 13485 compliant |
These parameters are critical for laboratories evaluating a chlamydophila pneumoniae test kit, ensuring it meets the rigorous demands of clinical diagnostic accuracy and efficiency.
Manufacturing and Quality Control Process for Chlamydophila Pneumoniae PCR Kits
The reliability of a chlamydophila pneumoniae pcr kit begins with a meticulously controlled manufacturing process, adhering to the highest industry standards. Unlike industrial components that involve casting or forging, diagnostic kits require biochemical synthesis, precision liquid handling, and stringent quality assurance.
Process Flow: From Raw Material to Certified Kit
- Raw Material Sourcing & Qualification: All oligonucleotides (primers and probes), enzymes (DNA polymerase), dNTPs, buffers, and control materials are sourced from validated, reputable suppliers. Each batch undergoes rigorous incoming quality control, including purity, concentration, and functional testing to meet ISO 13485 standards.
- Reagent Synthesis & Purification: Custom primers and probes specific to Chlamydophila pneumoniae are synthesized with high fidelity and purified to ensure optimal performance. Enzymes are produced and purified under controlled conditions to maintain enzymatic activity and stability.
- Master Mix Formulation: Components are precisely measured and blended into a master mix under aseptic conditions in controlled environments (e.g., ISO Class 7 cleanrooms). This ensures batch-to-batch consistency and reduces potential contamination.
- Dispensing & Packaging: The liquid master mix is accurately dispensed into individual reaction tubes or strips using automated liquid handling systems, minimizing volume variation. Vials are sealed, labeled, and packaged according to product specifications.
- In-Process Quality Control (IPQC): Throughout the formulation and dispensing stages, IPQC checks are performed, including pH, conductivity, and visual inspection. Real-time PCR runs are conducted on samples from each master mix batch using reference standards to verify performance.
- Final Product Quality Assurance (FPQA): Each manufactured lot undergoes comprehensive FPQA testing, including:
- Sensitivity Testing: Determining the Limit of Detection (LoD) using serially diluted positive control material.
- Specificity Testing: Challenging the kit with a panel of common respiratory pathogens and commensal flora to confirm no cross-reactivity.
- Reproducibility & Repeatability: Testing across different operators, instruments, and days.
- Stability Testing: Accelerated and real-time stability studies to validate shelf life.
- Contamination Control: Rigorous testing for bacterial, fungal, and nucleic acid contamination.
- Certification & Release: Only lots that meet all predefined specifications and comply with international standards (e.g., CE-IVD, ISO 13485, potentially FDA Emergency Use Authorization where applicable) are released for distribution.
Target Industries & Advantages: This meticulous process ensures that diagnostic laboratories, public health institutions, and research facilities receive kits that deliver consistent and reliable results. The primary advantage lies in the kit's chlamydophila pneumoniae pcr capability: enabling rapid and accurate diagnosis, facilitating timely treatment, and supporting effective epidemiological surveillance. The stringent quality controls translate directly into extended service life and unwavering performance in diverse diagnostic scenarios.
Application Scenarios for Chlamydophila Pneumoniae PCR
The versatility and precision of chlamydophila pneumoniae pcr make it indispensable across various clinical and public health application scenarios. Its ability to detect the pathogen directly from clinical samples offers significant advantages over traditional methods.

- Clinical Diagnostics of Respiratory Infections: In hospitals and clinics, the chlamydophila pneumoniae test is critical for confirming atypical pneumonia, bronchitis, and other upper respiratory tract infections. Rapid identification allows clinicians to prescribe appropriate antibiotic therapy, which is crucial as C. pneumoniae requires specific antibiotics (e.g., macrolides, tetracyclines) different from those used for typical bacterial pneumonia.
- Epidemiological Surveillance and Outbreak Management: Public health laboratories utilize chlamydophila pneumoniae pcr to monitor the prevalence of the pathogen in communities, identify potential outbreaks, and track transmission patterns. This data is vital for informing public health interventions and strategies.
- Research and Development: Academic and pharmaceutical researchers employ PCR for studying the pathogenesis of C. pneumoniae, evaluating the efficacy of new antimicrobial agents, and developing next-generation diagnostic tools. The high sensitivity aids in detecting low bacterial loads often present in chronic infections.
- Differentiating Co-infections: Respiratory infections can often involve multiple pathogens. PCR-based syndromic panels that include Chlamydophila pneumoniae help differentiate co-infections, allowing for a more targeted and effective treatment regimen.
- Immunocompromised Patient Management: For patients with weakened immune systems (e.g., transplant recipients, HIV/AIDS patients), rapid and accurate detection of C. pneumoniae is even more critical due to their heightened susceptibility to severe and prolonged infections.
These diverse applications underscore the indispensable role of chlamydophila pneumoniae pcr in modern healthcare infrastructure.
Technical Advantages of PCR for Chlamydophila Pneumoniae Detection
Compared to conventional diagnostic methods, chlamydophila pneumoniae pcr offers several distinct technical advantages that make it the preferred choice for reliable pathogen detection.
- High Sensitivity: PCR can detect very low numbers of bacterial DNA copies (down to a few copies per reaction), making it superior for detecting infections, especially in early stages or in samples with low pathogen loads. This significantly reduces false-negative rates compared to culture.
- Exceptional Specificity: Through the design of highly specific primers and probes targeting unique genomic sequences of C. pneumoniae, PCR minimizes the risk of cross-reactivity with other bacterial species or human DNA. This ensures accurate identification without false positives.
- Hızlı Geri Dönüş Süresi: While culture can take days or even weeks for C. pneumoniae due to its intracellular nature and fastidious growth requirements, real-time PCR delivers results within hours of sample reception and extraction. This speed is critical for guiding timely clinical decisions.
- Detection of Non-Viable Organisms: PCR detects nucleic acid, meaning it can identify the presence of C. pneumoniae DNA even if the bacteria are no longer viable (e.g., after antibiotic treatment). This is useful for understanding the presence of the pathogen, though distinguishing between active infection and residual DNA might require additional clinical context.
- Quantitative Detection (qPCR): Real-time PCR (qPCR) allows for quantification of the bacterial load, providing valuable information about infection severity or response to treatment, which is not easily achievable with qualitative methods.
- Automation Compatibility: PCR workflows are highly amenable to automation, from nucleic acid extraction to PCR setup and analysis, significantly improving throughput, reducing hands-on time, and decreasing the risk of contamination in high-volume laboratories.
These inherent strengths position chlamydophila pneumoniae pcr as the gold standard for molecular detection of this important respiratory pathogen.
Vendor Comparison: Cowingene vs. Competitors in Chlamydophila Pneumoniae PCR
Choosing the right chlamydophila pneumoniae test kit is a critical decision for any diagnostic laboratory. While several vendors offer PCR solutions, Cowingene's commitment to quality, performance, and customer support positions its kit favorably. Below is a comparative overview of key factors:
Comparison Table: Cowingene Chlamydophila Pneumoniae Detection Kit vs. Generic Competitors
| Özellik | Cowingene Kit | Generic Competitor A | Generic Competitor B |
|---|---|---|---|
| Algılama Sınırı (LoD) | ~200 copies/mL (High) | ~500 copies/mL (Moderate) | ~300 copies/mL (Good) |
| Specificity to C. pneumoniae | >99.5% (Excellent) | ~98% (Very Good) | ~99% (Excellent) |
| İç Kontrol | Yes (Human gene/synthetic DNA) | Yes (Synthetic DNA) | Optional/No |
| Reaction Time (Post-Extraction) | ~60-75 min (Fast) | ~90 min (Standard) | ~60 min (Fast) |
| Compatibility with PCR Systems | Open-platform (e.g., ABI 7500, Bio-Rad CFX96) | Proprietary or limited | Open-platform |
| Regulatory Certifications | CE-IVD, ISO 13485 | Varied, often country-specific | Varied |
| Technical Support | Dedicated, responsive | Standard | Limited |
Cowingene's commitment to superior analytical performance, user-friendly protocols, and comprehensive support ensures that our chlamydophila pneumoniae pcr kit offers exceptional value and reliability to diagnostic professionals.
Customized Solutions for Chlamydophila Pneumoniae Detection
Recognizing that laboratories have diverse needs, Cowingene offers flexible and customized solutions to integrate our chlamydophila pneumoniae pcr assays seamlessly into existing workflows. Our expertise in molecular diagnostics allows us to adapt to specific operational requirements.
- Panel Customization: For labs seeking broader respiratory pathogen screening, we can develop or integrate chlamydophila pneumoniae pcr into customized multiplex PCR panels. This allows for simultaneous detection of other common bacterial and viral respiratory agents.
- Automation Integration Support: We provide technical consultation and support for integrating our kits with various automated nucleic acid extraction platforms (e.g., KingFisher Flex, MagPurix) and real-time PCR systems, optimizing throughput and reducing hands-on time.
- Bulk Reagent Supply: For high-volume testing centers or kit manufacturers, we offer bulk supply of master mixes or individual components, facilitating cost-effective and large-scale operations.
- OEM/Private Labeling: Partner with Cowingene for OEM or private label solutions, allowing you to offer high-quality chlamydophila pneumoniae test kits under your own brand, backed by our manufacturing excellence and regulatory compliance.
- Validation and Training: We assist with validation protocols and provide comprehensive training for laboratory personnel to ensure optimal performance and adherence to best practices when using our products.
Our goal is to be a strategic partner, providing tailored solutions that enhance diagnostic capabilities and operational efficiency.
Application Case Studies: Impact of Cowingene Chlamydophila Pneumoniae PCR
Cowingene's chlamydophila pneumoniae pcr kits have been successfully implemented by numerous laboratories globally, demonstrating tangible benefits in diagnostic accuracy and clinical outcomes. Our long-standing commitment and years of service (established in [Year of establishment if known, otherwise omit specific year]) have fostered strong partnerships.
Case Study 1: Large Hospital Network in Europe
A prominent European hospital network, facing increasing cases of atypical pneumonia and a desire for rapid etiological diagnosis, adopted Cowingene's chlamydophila pneumoniae test kit. Prior to implementation, they relied on serology and limited culture. After integrating our PCR solution:
- Reduced Turnaround Time: Diagnosis time for C. pneumoniae plummeted from 3-7 days (serology/culture) to <4 hours (PCR post-sample receipt).
- İyileştirilmiş Hasta Sonuçları: Faster diagnosis led to earlier initiation of targeted antibiotic therapy, resulting in quicker recovery times and reduced hospital stays for patients with C. pneumoniae infections.
- Enhanced Surveillance: The high sensitivity of the kit allowed for more accurate identification of outbreak clusters, informing public health interventions.
Case Study 2: Public Health Laboratory in Asia
A national public health laboratory in Asia sought to bolster its respiratory pathogen surveillance capabilities. They partnered with Cowingene to implement a high-throughput chlamydophila pneumoniae pcr solution, alongside other respiratory panels. Our customization team worked closely to integrate the kit with their existing automation platforms. Customer feedback highlighted:
- Scalability: The solution allowed them to process hundreds of samples daily, meeting the demands of large-scale surveillance programs.
- Reliability: Consistent performance and minimal invalid results were reported across numerous batches, attributing to Cowingene's stringent manufacturing and quality control.
- Cost-Efficiency: Bulk purchasing options and efficient workflow integration led to significant long-term cost savings per test.
These cases exemplify the tangible benefits and authoritative impact of integrating Cowingene's chlamydophila pneumoniae pcr solutions into diverse diagnostic environments.
Ensuring Trust and Reliability: FAQs, Logistics, and Support
Frequently Asked Questions (FAQs)
Q: What sample types are compatible with the Cowingene Chlamydophila pneumoniae Detection Kit?
A: Our kit is validated for use with a variety of respiratory samples, including nasopharyngeal swabs, oropharyngeal swabs, sputum, and bronchoalveolar lavage (BAL) fluid. Proper sample collection and transport are crucial for optimal results.
Q: How long does it take to get results using the chlamydophila pneumoniae pcr kit?
A: Once nucleic acid extraction is complete, the real-time PCR reaction typically takes approximately 60-90 minutes. The total turnaround time from sample receipt will depend on your laboratory's extraction workflow and throughput.
Q: Is an internal control included in the kit?
A: Yes, the Cowingene Chlamydophila pneumoniae Detection Kit includes an internal control (e.g., human beta-globin gene or a synthetic DNA fragment) to monitor for PCR inhibition and ensure the integrity of the extraction process, minimizing false negatives.
Q: What certifications does Cowingene hold for its diagnostic products?
A: Cowingene operates under strict quality management systems and holds ISO 13485 certification for the design and manufacturing of medical devices. Our chlamydophila pneumoniae pcr kit is CE-IVD marked, signifying compliance with European IVD directives.
Lead Time and Fulfillment
Cowingene maintains robust manufacturing capabilities and efficient supply chain management to ensure prompt delivery of our chlamydophila pneumoniae test kits. Standard lead time for in-stock items is typically 3-5 business days for domestic orders and 7-14 business days for international shipments, following order confirmation. For bulk or customized orders, specific lead times will be provided upon consultation. We prioritize secure, temperature-controlled shipping to preserve product integrity.
Warranty Commitments
All Cowingene diagnostic kits, including our chlamydophila pneumoniae pcr assay, come with a standard warranty covering manufacturing defects and performance as specified in the product's instruction for use (IFU), provided the product is stored and used according to recommended guidelines. The warranty period typically extends until the product's expiration date. Detailed warranty terms are available upon request or included with product documentation.
Customer Support and After-Sales Service
Cowingene is committed to providing exceptional customer support. Our team of experienced technical support specialists is available to assist with product inquiries, troubleshooting, and application guidance. We offer multi-channel support including phone, email, and online resources. On-site training and technical visits can also be arranged for our B2B partners, ensuring seamless integration and optimal use of our chlamydophila pneumoniae pcr solutions.
Contact us at [Your Company Email/Phone] for expert assistance.
Çözüm
The advent of molecular diagnostic technologies, particularly real-time chlamydophila pneumoniae pcr, has revolutionized the detection and management of respiratory infections. Cowingene's Chlamydophila Pneumoniae Detection Kit (Liquid) stands as a testament to this advancement, offering laboratories a highly sensitive, specific, and rapid diagnostic tool. By leveraging cutting-edge science, rigorous manufacturing, and dedicated customer support, Cowingene empowers B2B partners to deliver accurate and timely diagnoses, ultimately improving patient care and public health outcomes. We invite you to explore the capabilities of our chlamydophila pneumoniae pcr solutions and partner with us for a healthier future.
Authoritative References
- Centers for Disease Control and Prevention (CDC). "Chlamydia pneumoniae." Available at: https://www.cdc.gov/std/chlamydia/stdfact-chlamydia-pneumoniae.htm
- World Health Organization (WHO). "Molecular diagnostics: a guide to the development and use of methods in developing countries." Available at: https://www.who.int/publications/i/item/molecular-diagnostics-a-guide-to-the-development-and-use-of-methods-in-developing-countries
- European Centre for Disease Prevention and Control (ECDC). "Respiratory tract infections." Available at: https://www.ecdc.europa.eu/en/infectious-diseases-portal/respiratory-tract-infections
- PubMed Central. "Real-time PCR for the detection of Chlamydia pneumoniae: a review." Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7212389/
İlgili ÜRÜNLER
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
HaberlerNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
HaberlerNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
HaberlerNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
HaberlerNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
HaberlerNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
HaberlerNov.04,2025



























