Sep . 13, 2025 10:40 Back to list
Diarrhea Kit for Rapid & Accurate PCR Panel Diagnosis
The Imperative of Advanced Diarrhea Diagnostics: Industry Trends and Innovations
Diarrhea remains a significant global health challenge, impacting millions annually across all age groups, with particularly severe consequences in pediatric and immunocompromised populations. The accurate and timely identification of etiologic agents is paramount for effective treatment, infection control, and public health surveillance. Traditional diagnostic methods, often reliant on culture or immunoassay, can be time-consuming, lack sensitivity, or fail to detect multiple pathogens simultaneously. This landscape has driven a robust demand for advanced molecular diagnostic solutions, particularly for comprehensive diarrhea pcr panels.
Current industry trends highlight a distinct shift towards multiplex PCR technologies. These panels offer the capability to screen for numerous bacterial, viral, and parasitic pathogens from a single clinical sample, significantly reducing turnaround time and improving diagnostic yield. The integration of automation and high-throughput capabilities further streamlines laboratory workflows, addressing the growing need for rapid diagnostics in both routine clinical settings and outbreak investigations. Innovations in lyophilized reagents and liquid formulations are also enhancing kit stability and ease of use, making sophisticated molecular testing more accessible to a broader range of laboratories.
The market for molecular gastroenterology diagnostics is experiencing substantial growth, fueled by increasing awareness of healthcare-associated infections, the rise of antibiotic resistance, and the continuous advancement in PCR technology. Leading laboratories and healthcare providers are actively seeking robust, reliable, and cost-effective solutions that can deliver actionable results efficiently. This demand underscores the critical role of a high-performance diarrhea kit in modern diagnostic paradigms.
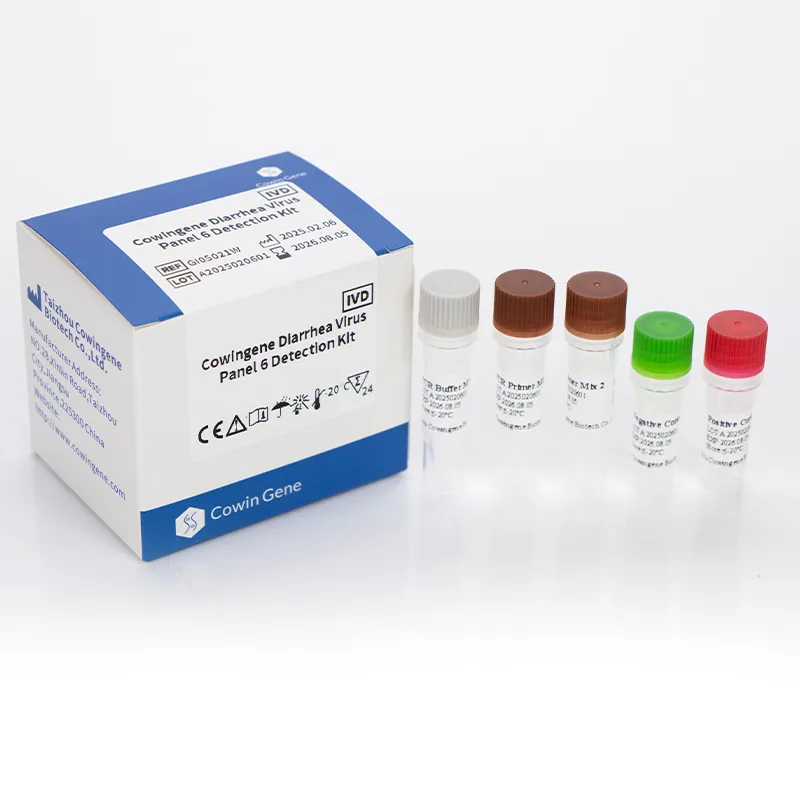
Technical Specifications of the Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid)
The Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid) represents a state-of-the-art solution for comprehensive viral pathogen detection in stool samples. This kit is meticulously engineered for multiplex diarrhea pcr analysis, targeting six common diarrheagenic viruses with high precision and sensitivity. The liquid formulation ensures optimal reagent stability and ease of pipetting, reducing potential errors in laboratory settings.
Key Technical Parameters
| Parameter | Specification |
|---|---|
| Product Name | Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid) |
| Target Pathogens | Norovirus GI, Norovirus GII, Rotavirus, Adenovirus, Sapovirus, Astrovirus |
| Detection Method | Real-time Multiplex PCR (RT-qPCR) |
| Sample Type | Stool samples |
| Specimen Volume | Requires minimal extracted nucleic acid volume |
| Limit of Detection (LoD) | Typically < 100 copies/mL for most targets (pathogen-specific) |
| Specificity | > 98% (verified against a comprehensive panel of endemic pathogens) |
| Sensitivity | > 95% (compared to reference methods) |
| Assay Time | Approximately 1.5 - 2 hours (post nucleic acid extraction) |
| Shelf Life | 12 months from manufacturing date (storage at -20°C) |
| Regulatory Status | CE-IVD marked (check specific region for local approval) |
The robust design of this diarrhea kit ensures reliable performance across various clinical settings, from high-volume hospital laboratories to public health surveillance centers. The precise targeting of multiple viral agents within a single reaction tube significantly enhances diagnostic throughput and reduces reagent consumption.
Manufacturing Process Flow of the Diarrhea Kit
The production of the Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid) adheres to stringent quality control protocols, ensuring the reliability and consistency of every batch. The manufacturing process is meticulously managed, from raw material procurement to final product assembly and packaging, reflecting the high standards expected for an IVD-certified diarrhea kit.
Schematic Steps of Production:
- Raw Material Sourcing & QC: High-grade oligonucleotides (primers and probes), enzymes (e.g., reverse transcriptase, Taq polymerase), buffers, and excipients are sourced from certified suppliers. Each raw material batch undergoes rigorous incoming quality control, including purity, concentration, and functional testing, to meet ISO 13485 standards.
- Oligonucleotide Synthesis & Purification: Custom DNA/RNA oligonucleotides are synthesized using advanced phosphoramidite chemistry. Post-synthesis, they are extensively purified (e.g., HPLC) to ensure high purity, critical for optimal PCR performance and specificity, minimizing non-specific amplification.
- Reagent Formulation & Mixing: Purified oligonucleotides, enzymes, dNTPs, and proprietary buffer components are precisely measured and mixed in controlled cleanroom environments. The liquid formulation requires meticulous attention to homogeneity and concentration accuracy to ensure consistent performance across all analytes in the multiplex panel.
- Filling & Aliquoting: The formulated master mix is aseptically aliquoted into individual reaction tubes or strips using automated liquid handling systems to prevent contamination and ensure precise volume dispense. This process maintains the integrity of the reagents and contributes to the kit's overall stability.
- Primary Packaging & Sealing: Aliquoted reagents are sealed in their primary container111s, typically PCR tubes or plates, which are designed for optimal thermal cycling and minimal evaporation. Each container111 is labeled with lot numbers and expiry dates.
- Final Kit Assembly: Primary packaged reagents are assembled with other kit components, such as positive controls, negative controls, and detailed instruction for use (IFU), into secondary packaging.
-
Finished Product Quality Control: Every manufactured lot undergoes comprehensive functional testing. This includes:
- Sensitivity Testing: Verification of the Limit of Detection (LoD) against known pathogen standards.
- Specificity Testing: Confirmation of non-reactivity with non-target pathogens and human genomic DNA.
- Reproducibility & Repeatability: Assessment of inter- and intra-assay variability.
- Stability Testing: Accelerated and real-time stability studies to validate stated shelf life (e.g., 12 months at -20°C).
- Labeling & Secondary Packaging: Kits are labeled with all required regulatory information, including CE-IVD marking, lot numbers, manufacturing date, expiry date, and storage conditions. Secure secondary packaging protects the kit during transit and storage.
Advantages in Application Scenarios:
- Rapid Turnaround: The streamlined manufacturing process ensures a stable and efficient product, enabling laboratories to deliver rapid diagnostic results, crucial for immediate patient management and outbreak response.
- High Accuracy: Rigorous QC and adherence to ISO standards guarantee high sensitivity and specificity, minimizing false positives and negatives, which is vital for patient trust and clinical decision-making.
- Ease of Use: The liquid format reduces the need for complex reconstitution steps, simplifying laboratory workflow and reducing hands-on time, a significant advantage in busy diagnostic settings.
- Cost-Effectiveness: Multiplex detection from a single sample reduces reagent usage and labor compared to multiple individual tests, optimizing laboratory resource allocation.
- Extended Shelf Life: Optimized formulation and packaging ensure a reliable 12-month shelf life, offering logistical flexibility for procurement and inventory management for laboratories.
Target industries for this advanced diarrhea kit include clinical diagnostic laboratories, hospital microbiology departments, public health institutions, and research facilities focused on infectious disease surveillance and epidemiology.
Application Scenarios and Technical Advantages
The Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid) is engineered for versatility and high performance across various clinical and public health applications, offering substantial technical advantages over traditional methods.
Typical Application Scenarios:
- Hospital and Clinical Laboratories: Used for rapid diagnosis of acute gastroenteritis, particularly in pediatric wards, emergency rooms, and intensive care units where swift pathogen identification impacts patient isolation, treatment, and resource allocation.
- Public Health Surveillance: Essential for identifying the causative agents during outbreaks in community settings, nursing homes, or cruise ships, enabling quick public health interventions and preventing further spread.
- Infection Control: Helps in differentiating between viral and bacterial causes of diarrhea, thereby guiding appropriate antibiotic stewardship and reducing unnecessary antibiotic use.
- Research and Epidemiology: Facilitates large-scale studies on viral gastroenteritis prevalence, seasonality, and strain typing, contributing to a deeper understanding of disease dynamics.
Technical Advantages:
- Multiplex Detection: Simultaneous detection of 6 key diarrheagenic viruses (Norovirus GI, Norovirus GII, Rotavirus, Adenovirus, Sapovirus, Astrovirus) in a single reaction, saving time and sample material. This capability distinguishes a robust diarrhea pcr panel from single-target tests.
- High Sensitivity and Specificity: Real-time PCR technology offers superior analytical sensitivity, detecting low viral loads that might be missed by less sensitive methods. Specific primers and probes minimize cross-reactivity, ensuring accurate identification.
- Reduced Turnaround Time (TAT): From sample to result (post-extraction), the entire assay can be completed in approximately 1.5-2 hours, providing clinicians with timely information for patient management.
- Liquid Reagent Format: Eliminates the need for reconstitution, simplifying workflow, minimizing pipetting errors, and reducing hands-on time. This enhances laboratory efficiency and reduces operator variability.
- Internal Control: Includes an internal control (IC) to monitor the entire PCR process, from nucleic acid extraction to amplification, ensuring the validity of negative results and detecting potential PCR inhibition.
- Compatibility: Designed to be compatible with common real-time PCR instruments found in clinical laboratories, facilitating easy integration into existing laboratory setups.
- Improved Patient Outcomes: Rapid and accurate diagnosis allows for targeted therapy, appropriate infection control measures, and reduced length of hospital stays, ultimately improving patient care.
Vendor Comparison: Cowingene vs. Competitors in Diarrhea PCR Panels
In the rapidly evolving market of molecular diagnostics, the choice of a diarrhea pcr panel is critical for laboratories. A comparative analysis highlights the distinctive strengths of the Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid) against other prominent solutions.
Competitive Landscape Overview:
While many companies offer molecular diagnostic kits for gastroenteritis, key differentiators often lie in the breadth of pathogen detection, assay format, turnaround time, and overall cost-efficiency. Competitors typically include established players offering comprehensive syndromic panels or more focused viral panels. These often vary in terms of target organisms (some include bacteria, parasites, and viruses, while others focus on a subset), sample types, and the required instrumentation.
Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid) vs. Selected Competitor (Illustrative):
| Feature | Cowingene Diarrhea Virus Panel 6 (Liquid) | Competitor X (Lyophilized Viral Panel) |
|---|---|---|
| Target Pathogens | Norovirus GI, Norovirus GII, Rotavirus, Adenovirus, Sapovirus, Astrovirus (6 viral targets) | Norovirus GI/GII, Rotavirus, Adenovirus, Sapovirus (4 viral targets, or wider panel with bacterial/parasitic) |
| Reagent Format | Liquid (ready-to-use master mix) | Lyophilized (requires reconstitution) |
| Hands-on Time | Low (no reconstitution, minimal pipetting) | Moderate (reconstitution steps add time/risk) |
| Assay Time (post-extraction) | ~1.5 - 2 hours | ~2 - 2.5 hours |
| LoD (typical) | < 100 copies/mL (target-specific) | < 150 copies/mL (target-specific) |
| Internal Control | Yes (included) | Yes (included, sometimes external) |
| Cost per Test | Competitive, optimized for viral panel focus | Varies, potentially higher if broader syndromic panel |
| Ease of Automation | High (liquid format integrates easily with robotics) | Moderate (reconstitution steps add complexity for automation) |
Cowingene's liquid formulation significantly reduces hands-on time and the potential for errors associated with lyophilized reagents. This, coupled with its broad viral detection panel and competitive turnaround time, positions the Cowingene diarrhea kit as a compelling choice for laboratories prioritizing efficiency and accuracy in viral gastroenteritis diagnostics.
Customized Solutions and Application Case Studies
Cowingene understands that diverse laboratory needs often extend beyond standard product offerings. We provide flexible solutions, including customization options and dedicated support, tailored to meet specific research or diagnostic requirements for our diarrhea kit.
Customization Options:
- Panel Configuration: While the standard kit targets 6 viral pathogens, Cowingene can explore options for modifying panel targets to include or exclude specific viruses based on regional prevalence or research focus (subject to feasibility and volume).
- Packaging Formats: Custom packaging, including different reaction tube configurations or plate formats, can be arranged to integrate seamlessly with specific laboratory automation platforms.
- Volume and Scale: Tailored bulk packaging or specific assay sizes can be developed for high-throughput laboratories or large-scale epidemiological studies, optimizing cost and workflow.
- Validation Support: Collaborative efforts for validation against specific in-house sample cohorts or regulatory requirements can be provided to ensure smooth implementation.
Application Case Study: Regional Public Health Laboratory
Challenge: A regional public health laboratory in Southeast Asia faced challenges in rapidly identifying causative viral agents during seasonal gastroenteritis outbreaks. Their previous methods were labor-intensive and often missed co-infections, leading to delayed public health interventions and ineffective patient management. They needed a high-throughput, reliable diarrhea pcr panel.
Solution: The laboratory implemented the Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid). Due to high sample volumes, Cowingene provided customized bulk packaging of the liquid reagents and assisted with protocol optimization for their automated nucleic acid extraction system.
Results:
- Reduced average turnaround time for viral identification from 2-3 days to under 8 hours.
- Increased detection rate of viral pathogens by 25%, particularly for co-infections and less common viruses like Sapovirus and Astrovirus.
- Enabled rapid deployment of public health measures, including targeted sanitation campaigns and improved patient isolation protocols in healthcare facilities.
- Significant reduction in reagent waste and hands-on time due to the liquid format and bulk packaging, leading to an estimated 15% cost saving on reagents and labor.
This case study exemplifies the practical benefits of integrating a high-performance diarrhea kit like Cowingene's into routine diagnostics and outbreak management strategies. The ease of use, coupled with its robust analytical performance, makes it an invaluable tool for public health and clinical laboratories globally.

Ensuring Trust: FAQ, Lead Time, Warranty, and Support
At Cowingene, we are committed to building long-term partnerships based on trust, reliability, and exceptional customer service. Our comprehensive approach to client support underpins the quality of our Diarrhea Virus Panel 6 Detection Kit (Liquid).
Frequently Asked Questions (FAQ):
- Q: What instruments are compatible with the Cowingene Diarrhea Virus Panel 6 Detection Kit?
A: The kit is designed for compatibility with most standard real-time PCR instruments equipped with appropriate detection channels (e.g., FAM, VIC/HEX, ROX, Cy5). Please contact our technical support for a detailed list of validated instruments. - Q: How does the liquid format impact shipping and storage?
A: The liquid format requires shipment on dry ice and storage at -20°C. This ensures the integrity and stability of the reagents throughout their 12-month shelf life. - Q: Can this kit detect bacterial or parasitic causes of diarrhea?
A: This specific kit is designed to detect 6 common diarrheagenic viruses only. For bacterial or parasitic detection, we offer separate or combination panels, which can be discussed with our sales team. - Q: Is nucleic acid extraction required before using the kit?
A: Yes, nucleic acid extraction from stool samples is a prerequisite for PCR. The kit is compatible with most commercially available nucleic acid extraction methods.
Lead Time and Fulfillment:
Standard lead time for the Cowingene Diarrhea Virus Panel 6 Detection Kit (Liquid) is typically 2-4 weeks from order confirmation, depending on order size and current inventory. For urgent requests or large-volume orders, we encourage direct communication with our sales department to discuss expedited shipping and customized fulfillment schedules. We maintain robust supply chain management to ensure consistent product availability.
Warranty Commitments:
Cowingene provides a comprehensive warranty for the Diarrhea Virus Panel 6 Detection Kit (Liquid), guaranteeing its performance according to the specifications outlined in the product's Instruction for Use (IFU) until the indicated expiry date, when stored and used as directed. In the unlikely event of a product defect or failure to meet performance specifications, we commit to prompt investigation and replacement or credit. Our warranty policy is compliant with all relevant regulatory standards, including CE-IVD requirements.
Customer Support Information:
Our dedicated technical support team comprises experienced molecular biologists and application specialists ready to assist with assay setup, troubleshooting, and interpretation of results. Support is available via phone and email during business hours. For complex issues or on-site assistance, our field application scientists can provide specialized support and training. Cowingene is committed to ensuring our clients achieve optimal results with our products.
- Email: support@cowingene.com
- Phone: +86-XXX-XXXX-XXXX (placeholder)
- Website: www.cowingene.com
Authoritative References
- ISO 13485:2016 Medical devices — Quality management systems — Requirements for regulatory purposes. International Organization for Standardization.
- Centers for Disease Control and Prevention. (2023). Norovirus: Clinical & Laboratory. Retrieved from cdc.gov/norovirus/hcp/clinical-lab-info.html
- European In Vitro Diagnostic Medical Device Regulation (IVDR) (EU) 2017/746. Official Journal of the European Union.
- Pang, X. L., & Preiksaitis, J. K. (2014). PCR-based detection of viral gastroenteritis pathogens. Clinical Microbiology Reviews, 27(3), 603-643.
- World Health Organization. (2023). Diarrhoeal disease. Retrieved from who.int/news-room/fact-sheets/detail/diarrhoeal-disease
Related PRODUCTS
-
Understanding Monkeypox Testing PCR – Global Health & Diagnostic Insights
NewsNov.24,2025 -
Comprehensive Guide to Monkey Pox Detection: Methods, Applications & Innovations
NewsNov.23,2025 -
Essential Guide to Monkeypox Detection: Technologies, Applications & Future Trends
NewsNov.23,2025 -
Understanding Strep B Test Cost: Global Insights and Healthcare Impact
NewsNov.22,2025 -
Group B Strep DNA Test – Fast, Accurate Screening to Prevent Neonatal Infection
NewsNov.21,2025 -
Essential Guide to Group B Strep Test Kits: Benefits, Uses & Innovations
NewsNov.20,2025







