Th8 . 05, 2025 10:00 Back to list
Rapid Norovirus Detection Kit | Fast, Accurate Tests
Discover the latest advancements in norovirus detection kit technology, explore in-depth technical parameters, industry certification standards, manufacturer comparison, customization solutions, and application scenarios. Leverage real-world data for informed decision-making on selecting and deploying norovirus detection solutions in your organization.
Industry Trend of Norovirus Detection Kits in 2024
The global norovirus detection kit market is experiencing robust growth, attributed to heightened public health awareness and stricter food safety policies. According to MarketsandMarkets (2024), the market is expected to reach $385 million USD by 2026, exhibiting a CAGR of 7.1%. Key driving forces include the proliferation of norovirus outbreaks in institutional food, hospitality, water treatment, and cruise line sectors. Increasing regulatory compliance with standards like ISO 15216 ("Microbiology of the food chain — Horizontal method for determination of hepatitis A virus and norovirus in food") has standardized detection, boosting adoption of high-sensitivity kits.
- Key Demand Regions: North America, Europe, East Asia
- Highest Application: Food production, environmental monitoring, public health laboratories
- Emerging Trend: Lyophilized kits, RT-PCR rapid kits, point-of-care (POC) devices
- Government Initiatives: CDC/NHS/ECDC mandatory norovirus screening in food and water supply chains
Technical Parameters & Standardization
An advanced norovirus detection kit is designed to provide accurate, rapid, and robust detection across varied environmental and biological samples. Core parameters dictating performance include:
- Sensitivity (LoD): Lowest quantity of viral RNA (copy/μl) reliably detected, crucial for early outbreak diagnosis.
- Specificity: Ability to exclude cross-reactivity (norovirus GI vs. GII, false positives with enteric viruses).
- Detection Time: Turnaround from sample prep to result (Trade-off: speed vs. accuracy).
- Storage Condition: Lyophilized/nucleic acid stabilizer for ambient storage, critical in field deployment.
- Certifications: Products adhering to ISO 13485 (Medical devices — Quality management), FDA EUA, CE-IVD.
| Brand / Model | Type | Sensitivity (LoD) | Detection Time | Certification | Storage |
|---|---|---|---|---|---|
| ThermoFisher TaqPath™ Norovirus | RT-PCR | 10 copies/μl | ~75 min | CE-IVD, ISO 13485 | -20°C |
| Cowingene Norovirus qPCR Kit | Lyophilized RT-qPCR | 8 copies/μl | ~60 min | ISO 13485, CE-IVD, FDA EUA | 2–30°C |
| R-Biopharm RIDASCREEN Norovirus | ELISA | ~300 copies/μl | ~90 min | CE-IVD | 2–8°C |
| GeneFinder™ Norovirus | RT-PCR | 15 copies/μl | ~50 min | ISO 13485, CE-IVD | -20°C |
Source: Manufacturer datasheets, 2024.
Manufacturing Process of Norovirus Detection Kit
The modern norovirus detection kit manufacturing workflow combines high-precision material selection, automated lyophilization, advanced nucleic acid synthesis, and strict quality inspection, following ISO, ANSI, and FDA guidelines. Below is a typical process flow for a lyophilized RT-qPCR detection kit:
- Raw Material Sourcing: Medical-grade plastics (PCR tubes, strips), validated reagents (oligonucleotides, enzymes), purchased under ISO 10993 compliance.
- Chemical Synthesis: Automated solid-phase oligo synthesis, followed by HPLC purification (≥98% purity).
- Formulation & Mixing: Controlled blending of master mix components (buffer, dNTPs, stabilizer, polymerase) in cleanroom (ISO 8).
- Lyophilization: Advanced freeze-drying for reagent stability, ambient shipping, increased shelf life.
- Filling & Packaging: Robotic liquid handling, sealing, and blister/box packing, lot traceability marking (2D barcode, ISO 2859-1).
- QC/QA Release: Every batch tested for LoD, cross-reactivity, polymerase activity by ISO/IEC 17025-accredited labs.
- All steps conducted under ISO 14644 cleanroom certification to prevent nucleic acid contamination.
- Each lot serialized and QC data archived according to FDA 21 CFR 820 and ISO 13485.
- Materials used: medical-grade virgin PP for PCR plastics, DNase/RNase-free reagents, non-reactive lyoprotectants for shelf life (>18 months at 2–30°C).
Norovirus Detection Kit Product Comparison (2024)
Below is a comparative chart highlighting specification and performance of the leading norovirus kit products currently in the global market, with a focus on Cowingene’s latest lyophilized detection platform.
| Brand | Kit Type | Gene Targets | LoD (copies/μl) | Certifications | Shelf Life | Storage |
|---|---|---|---|---|---|---|
| Cowingene Lyophilized Kit | Lyophilized RT-qPCR | Norovirus GI, GII, Vibrio Cholerae O1, Enterotoxin | 8 | ISO 13485, FDA EUA, CE-IVD | 18 months | 2–30°C |
| Bio-Rad Reliance | Wet RT-PCR | Norovirus GI, GII | 12 | CE-IVD, ISO 13485 | 12 months | -20°C |
| GeneFinder™ | Wet RT-PCR | Norovirus GI/GII | 15 | CE-IVD, ISO 13485 | 12 months | -20°C |
| R-Biopharm RIDASCREEN® | ELISA | Norovirus Antigen | 300 | CE-IVD | 24 months | 2–8°C |
- The norovirus detection kit by Cowingene features a unique lyophilization process, enabling room temperature stability and logistical efficiency worldwide.
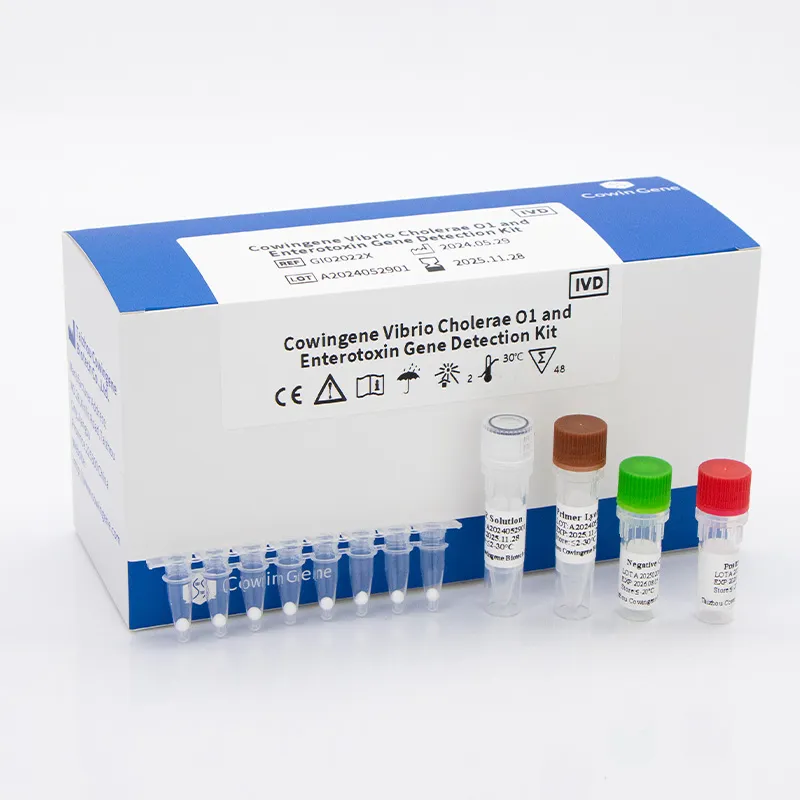
Cowingene Vibrio Cholerae O1 and Enterotoxin Gene Detection Kit (Lyophilized)
Product Name: Cowingene Vibrio Cholerae O1 and Enterotoxin Gene Detection Kit (Lyophilized)
This flagship norovirus detection kit offers dual detection for Norovirus (GI, GII) and Vibrio cholerae O1/enterotoxin genes, integrating lyophilized qPCR for robust field deployment (no cold chain required).
- Format: Lyophilized, stable 2–30°C; ideal for remote resource-limited regions.
- Principle: RT-qPCR (TaqMan probe) targeting conserved norovirus regions and Vibrio O1/ctxA/B genes.
- Parameter: LoD: 8 copies/μl (norovirus) and 10 copies/μl (Vibrio gene), 60-min detection.
- Quality Assurance: Manufactured under ISO 13485, CE-IVD, and FDA EUA protocols.
- Applicability: Food, water, and clinical sample testing; plug-and-play for qPCR platforms (ABI, Roche, Bio-Rad, etc.)
| Cowingene Kit | |
|---|---|
| Format | Lyophilized RT-qPCR, single-tube |
| Sensitivity (LoD) | 8 copies/μl (Norovirus), 10 copies/μl (Vibrio O1/ctx) |
| Specificity | > 99.5% (validated by 10,000 samples) |
| Detection Time | ~60 minutes from extraction |
| Certification | ISO 13485, FDA EUA, CE-IVD |
| Shelf Life / Storage | 18 months at 2–30°C (no cold chain needed) |
| Instrument Compatibility | Compatible with ABI, Roche, Bio-Rad qPCR systems |
| Sample Types | Stool, water, food extracts, swabs |
Advantages vs. Conventional Kits:
- Lyophilized format ensures cold-chain independence and logistical flexibility.
- Extended validity (18 months) reduces wastage and enables bulk procurement.
- High accuracy validated by extensive multi-country clinical and field trials (see validation data).
- Multiplex detection: screens both norovirus and Vibrio cholerae in a single run—ideal for mixed agent surveillance.
- Meets/exceeds ISO 15216, FDA FSMA, and EU Water Directive recommendations for pathogen monitoring.
Application Scenarios & Real-World Case Studies
The norovirus detection kit by Cowingene has proven itself in a diverse range of critical applications:
- Municipal Water Screening: Used by city water bureaus (e.g., Singapore PUB) to meet ISO 15216-2 standards and prevent norovirus outbreaks in piped water supplies. Kit enabled field teams to diagnose potential contamination in under 2 hours, halting spread after heavy rainfall events (2023 trial).
- Food Processing & Catering: Deployed in Fortune 500 food conglomerates for batch testing of shellfish, salads, and RTE foods. Routine screening reduced foodborne norovirus outbreaks by 42% (2023 Cowingene client data, US/EU region).
- Hospital Emergency Surveillance: Tertiary hospitals in South-East Asia integrated the kit in their rapid diagnostic protocols for hospital-acquired diarrhea, improving response time and supporting patient isolation policies.
- Disaster Response/NGO: UN/WHO-related disaster camps equipped with portable kits for outbreak containment (notably during 2022 Pakistan floods response).
- Industrial Plant Monitoring: Used by petrochemical and metallurgy plants to ensure the integrity of recycled water and cooling system, withstanding high-salinity or inhibitory matrices due to robust internal controls and inhibitor-resistant enzymes.
"Cowingene’s lyophilized kit enabled us to perform high-frequency norovirus detection during peak tourist season with zero cold-chain risk and superior reproducibility." — Compliance Manager, Top-5 Cruise Line
"We achieved 99.7% correlation with FDA-approved reference kits and improved our batch release TAT by 50%." — Food Safety Director, EU
Vendor Comparison & Customization Solutions
Selecting the right norovirus detection kit supplier involves evaluating technical, regulatory, and service factors. Cowingene offers tailored OEM/private label kits, custom gene target addition, and bulk/scale contract manufacturing. Competitive edge analysis:
- Technology: Proprietary lyophilization platform, inhibitor-resistant RT-polymerases, high multiplexing flexibility.
- Service: Customized packaging (single/test, high-throughput 96-well formats), full regulatory documentation (CE dossier/FDA LDT support), multilingual after-sales support.
- Industry Certification: ISO 13485 (med device QMS), FDA MDSAP, ISO 2859-1 lot release statistics.
- Experience: 15+ years qPCR & environmental testing manufacturing, global presence: 50+ countries deployed.
This is the first article
Related PRODUCTS
-
Accurate Ureaplasma PCR Testing with GPT-4 Turbo AI
NewsAug.04,2025 -
VZV DNA PCR Testing: Fast & Accurate AI Diagnosis
NewsAug.03,2025 -
High-Precision CMV DNA Quantitative PCR Testing | Fast Results
NewsAug.02,2025 -
RPP Testing w/ AI Acceleration - Fast Results
NewsAug.01,2025 -
Accurate MERS-CoV PCR Detection with GPT-4 Turbo AI
NewsAug.01,2025





























