أغسطس . 14, 2025 04:20 Back to list
Advanced Respiratory Panel Lab Test for Quick Diagnosis
Understanding the Critical Role of Respiratory Panels in Modern Diagnostics
In an era of evolving public health challenges, the rapid and accurate identification of respiratory pathogens is paramount. A comprehensive panel respiratory system provides a robust solution, enabling healthcare professionals and public health authorities to make informed decisions swiftly. These advanced diagnostic tools are engineered to detect multiple pathogens simultaneously, significantly improving clinical workflow efficiency and patient outcomes. From distinguishing common colds from influenza to identifying novel viral threats, the utility of an integrated respiratory panel is undeniable.
The demand for efficient and reliable diagnostic solutions has surged, driven by global health crises and the imperative for early disease management. A state-of-the-art respiratory panel streamlines the diagnostic process, offering a clear pathway for targeted treatments and effective infection control strategies within clinical and laboratory settings. This comprehensive approach is vital for both individual patient care and broader epidemiological surveillance, providing critical insights into community health trends.
Industry Trends and Technological Advancements in Respiratory Diagnostics
The landscape of respiratory diagnostics is characterized by continuous innovation. Key trends include the shift towards multiplex PCR assays, which enable simultaneous detection of a broad spectrum of viruses and bacteria from a single sample. This minimizes turnaround time and conserves precious sample material. Another significant trend is the increasing adoption of lyophilized reagents, offering enhanced stability, simplified shipping and storage logistics, and reduced risk of contamination. According to a report by Grand View Research, the global molecular diagnostics market size was valued at USD 24.3 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 9.2% from 2023 to 2030, largely driven by the rising prevalence of infectious diseases and technological advancements in diagnostic platforms.
Furthermore, the integration of automation and bioinformatics in respiratory panel lab workflows is revolutionizing throughput and data analysis. This allows for higher sample volumes to be processed with greater accuracy, reducing human error and accelerating results. Point-of-care testing (POCT) solutions are also gaining traction, bringing diagnostic capabilities closer to the patient, particularly in remote areas or emergency settings. These trends collectively underscore the industry's commitment to delivering faster, more accessible, and more comprehensive diagnostic insights for respiratory illnesses.
Comprehensive Workflow of a Respiratory Diagnostic Panel Lab
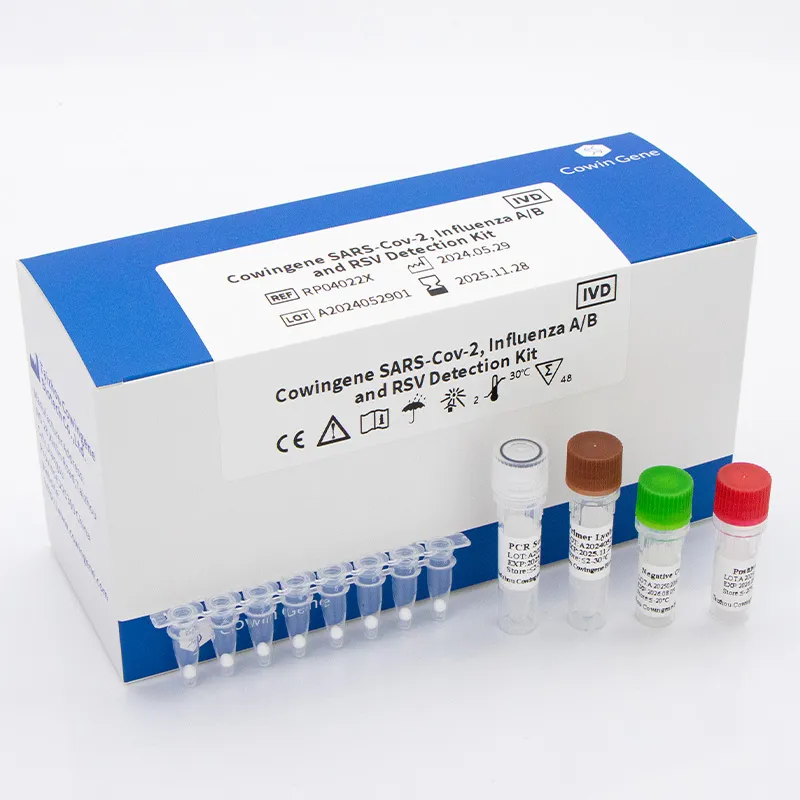
The efficient operation of a respiratory panel diagnostic lab involves a meticulous multi-step process, from sample collection to result interpretation, ensuring high accuracy and reliability. This section details the typical workflow, highlighting key stages and quality control measures crucial for delivering precise diagnostic outcomes, especially when utilizing advanced kits like the Cowingene SARS-Cov-2, Influenza A/B and RSV Detection Kit (Lyophilized).
- Sample Collection & Preparation: Nasopharyngeal or oropharyngeal swabs, saliva, or sputum samples are collected, then transported in viral transport media (VTM). Proper sample handling and storage are critical to maintain RNA/DNA integrity.
- Nucleic Acid Extraction: Automated or manual methods are used to extract viral RNA from the collected samples. The purity and concentration of the extracted nucleic acid are verified, ensuring optimal input for downstream amplification.
- Reverse Transcription & Amplification: This is where the core of the diagnostic panel comes into play. For RNA viruses, reverse transcription (RT) synthesizes cDNA, which is then amplified using real-time PCR (qPCR). Kits like the Cowingene detection kit, using lyophilized reagents, provide a stable and precise mixture for this critical step, targeting specific genes for SARS-CoV-2, Influenza A/B, and RSV. The lyophilized format ensures long-term stability and simplifies reagent preparation, significantly reducing hands-on time and potential errors.
- Detection Standards & Quality Control: Adherence to international standards such as ISO 13485 for medical devices and CLIA (Clinical Laboratory Improvement Amendments) regulations in the US, along with CE-IVD marking for European markets, is paramount. Internal and external quality controls are run with every batch to monitor assay performance, detect inhibition, and ensure result validity. Regular proficiency testing programs further validate the lab's accuracy.
- Data Analysis & Interpretation: PCR amplification curves are analyzed by specialized software. Threshold cycles (Ct values) indicate the viral load. Results are interpreted based on validated cut-offs and reported to clinicians.
- Reporting & Archiving: Secure electronic systems are used for reporting results, often integrated with Electronic Health Records (EHR) systems. Comprehensive records are archived for regulatory compliance and epidemiological tracking.
The Cowingene SARS-Cov-2, Influenza A/B and RSV Detection Kit (Lyophilized) exemplifies the kind of advanced product material and precise manufacturing process (lyophilization under aseptic conditions) that ensure a long service life (typically 12-24 months from manufacture) and high reliability. These panels are primarily applicable in clinical diagnostic laboratories, public health surveillance centers, and research institutions, proving indispensable for rapid pathogen identification and outbreak management.
Key Technical Parameters of Advanced Respiratory Panels
Selecting the right respiratory panel requires a thorough understanding of its technical specifications. These parameters directly impact the diagnostic accuracy, efficiency, and suitability for various lab environments. The following table provides a comparative overview of critical technical specifications relevant to modern multiplex respiratory panels, including insights into products like the Cowingene SARS-Cov-2, Influenza A/B and RSV Detection Kit.
| Parameter | Description | Typical Range for High-Quality Panels | Cowingene Kit Specifics (Example) |
|---|---|---|---|
| Pathogens Detected | Number and type of viral/bacterial targets. | 3-20+ targets (e.g., Flu A/B, RSV, SARS-CoV-2, Adenovirus, etc.) | SARS-CoV-2, Influenza A, Influenza B, RSV (4 targets) |
| Sample Type | Suitable biological specimens for testing. | Nasopharyngeal swabs, Oropharyngeal swabs, Sputum, BALF | Nasopharyngeal swabs, Oropharyngeal swabs, Sputum |
| Turnaround Time (TAT) | Time from sample input to result generation. | 1-4 hours (from extraction to result) | Approx. 75 minutes after nucleic acid extraction |
| Sensitivity | Ability to correctly identify positive cases (lower LOD is better). | LOD < 200 copies/mL; >95% sensitivity | SARS-CoV-2: 100 copies/mL; Flu A/B: 50 copies/mL; RSV: 50 copies/mL |
| Specificity | Ability to correctly identify negative cases (< false positives). | >98% specificity | >99% specificity (no cross-reactivity with common respiratory pathogens) |
| Kit Stability/Shelf Life | Duration for which the kit maintains performance. | 12-24 months (room temperature for lyophilized) | 12 months at room temperature (2-30°C) |
| Required Equipment | Compatible PCR instruments and extraction systems. | Standard qPCR instruments (e.g., ABI 7500, Bio-Rad CFX96) | Standard Real-Time PCR Systems (e.g., Applied Biosystems 7500, Bio-Rad CFX96, SLAN-96P) |
Technical Advantages and Application Scenarios
The technical advantages of a well-designed respiratory panel lab solution are multifaceted, directly impacting operational efficiency and diagnostic confidence. Products like the Cowingene SARS-Cov-2, Influenza A/B and RSV Detection Kit (Lyophilized) offer superior benefits due to their innovative design. The lyophilized format eliminates the need for cold chain logistics, significantly reducing shipping costs and storage complexity, while also ensuring long-term reagent stability and extended shelf life. This leads to reduced waste and improved accessibility in diverse geographical locations. Moreover, the multiplexing capability provides rapid differential diagnosis, saving critical time in clinical settings compared to sequential single-target tests.
The broad application scenarios for these advanced respiratory panels include:
- Clinical Diagnostics: Essential for rapid and accurate diagnosis of respiratory infections in hospitals, clinics, and urgent care centers, facilitating prompt patient isolation and targeted antiviral/antibiotic treatment.
- Public Health Surveillance: Critical for monitoring seasonal outbreaks (e.g., influenza, RSV) and emerging viral threats, providing data for epidemiological studies and public health interventions. This allows authorities to track disease prevalence and spread effectively.
- Research & Development: Used in academic and pharmaceutical research for studying pathogen dynamics, vaccine efficacy trials, and antiviral drug development. The high sensitivity and specificity support robust scientific investigations.
- Emergency Preparedness: Integral to a country's readiness for pandemics or significant infectious disease events, enabling mass testing capabilities and rapid response.
Manufacturer Comparisons and Tailored Solutions
When evaluating providers for a comprehensive panel respiratory solution, discerning buyers must consider not just product specifications but also the manufacturer's commitment to quality, support, and innovation. While many companies offer molecular diagnostic kits, Cowingene distinguishes itself through its focus on lyophilized reagents, stringent quality control, and robust after-sales support. Unlike some competitors that may require extensive cold chain infrastructure or complex reagent preparation, Cowingene's lyophilized kits simplify workflows, reduce shipping costs, and minimize environmental impact. This streamlined approach offers significant operational savings and increased convenience for laboratories worldwide.
Cowingene also offers tailored solutions to meet specific laboratory needs. For instance, laboratories with unique throughput requirements or specific pathogen surveillance programs can consult with our technical team to explore customized packaging, bulk orders, or integration advice for existing automation platforms. Our dedicated support team works closely with clients to understand their operational challenges and provide optimized solutions, ensuring seamless adoption and maximum efficiency of our respiratory panel products.
Application Cases and Client Success Stories
The practical impact of a reliable respiratory panel is best illustrated through real-world applications. Cowingene's detection kits have been instrumental in various settings:
- Case Study 1: Regional Hospital Diagnostic Lab
A large regional hospital laboratory adopted Cowingene's SARS-Cov-2, Influenza A/B and RSV Detection Kit to manage peak respiratory season testing. Prior to implementation, they struggled with high sample volumes and differentiating co-circulating viruses, leading to delays. Post-adoption, the hospital reported a 30% reduction in turnaround time for multiplex respiratory testing and a significant decrease in inconclusive results. This allowed for faster patient triage and more effective allocation of resources. - Case Study 2: Public Health Surveillance Initiative
During a recent influenza season, a public health agency in Southeast Asia utilized our respiratory panel lab solutions for community-wide surveillance. The kit's high sensitivity and ease of use, even in remote field laboratories, enabled rapid identification of circulating strains, providing crucial data for vaccine efficacy monitoring and informing public health advisories. The lyophilized format was particularly beneficial for deployment in areas with limited cold chain infrastructure. - Client Feedback: "The Cowingene kit's stability and consistent performance have revolutionized our lab's efficiency, especially during high-demand periods. The lyophilized format is a game-changer for our logistics," commented Dr. Lena Singh, Head of Molecular Diagnostics at a leading research institution.
Trust and Support: Your Partner in Respiratory Diagnostics
Cowingene is committed to being a trusted partner in advanced diagnostic solutions. Our adherence to stringent quality standards, including ISO 13485 certification for medical device manufacturing, underscores our dedication to product excellence. We believe in transparency and comprehensive support for our clients.
Frequently Asked Questions (FAQ)
- Q: What is the shelf life of the Cowingene lyophilized kit?
A: The Cowingene SARS-Cov-2, Influenza A/B and RSV Detection Kit (Lyophilized) has a shelf life of 12 months when stored at room temperature (2-30°C). - Q: Does the kit require special equipment for use?
A: The kit is compatible with standard real-time PCR systems such as Applied Biosystems 7500, Bio-Rad CFX96, and SLAN-96P, making it easily integrable into most molecular diagnostic labs. - Q: What certifications does the kit hold?
A: The kit is CE-IVD marked, indicating compliance with European In Vitro Diagnostic Device Regulations, and has received Emergency Use Authorization (EUA) in various regions, demonstrating its reliability and performance.
Delivery Cycle and Warranty
We understand the urgency in diagnostics. Our standard delivery cycle for stock items is typically 3-5 business days domestically and 7-14 business days internationally, depending on the destination and customs clearance. For larger orders or custom solutions, lead times will be communicated clearly upon order confirmation. All Cowingene products, including our respiratory panel kits, come with a manufacturer's warranty covering defects in materials and workmanship, ensuring your investment is protected.
Dedicated Customer Support
Our commitment extends beyond product delivery. Cowingene provides comprehensive customer support, including technical assistance for assay setup, troubleshooting, and result interpretation. Our team of experienced specialists is available to ensure your laboratory achieves optimal performance with our diagnostic solutions. We offer online resources, training modules, and direct consultation services to empower your team.
References
- Grand View Research. Molecular Diagnostics Market Size, Share & Trends Analysis Report By Product (Reagents, Instruments, Services), By Application (Infectious Diseases, Oncology), By Technology, By End-use, By Region, And Segment Forecasts, 2023 - 2030.
- World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020.
- Centers for Disease Control and Prevention. Multiplex Real-Time RT-PCR Assay for Detection of SARS-CoV-2, Influenza A and B, and Respiratory Syncytial Virus (RSV).
Related PRODUCTS
-
Fast & Accurate Dengue PCR Test for Early Diagnosis
NewsAug.21,2025 -
Accurate Legionella Pneumophila Detection PCR Kits
NewsAug.19,2025 -
Rapid & Accurate PCR Influenza A Testing Solutions
NewsAug.18,2025 -
Accurate HPV Genotyping Test Kit | Home Self-Sampling
NewsAug.17,2025 -
Accurate Dengue PCR Test | Rapid RNA & RT-PCR Detection
NewsAug.15,2025