أغسطس . 13, 2025 04:00 Back to list
PCR Varicella Zoster Virus: Fast, Accurate Detection
The Evolving Landscape of Varicella-Zoster Virus Diagnostics
Varicella-Zoster Virus (VZV), a ubiquitous human alphaherpesvirus, is the causative agent of varicella (chickenpox) and herpes zoster (shingles). Accurate and timely diagnosis of VZV infections is paramount for effective patient management, particularly in immunocompromised individuals where the infection can lead to severe and life-threatening complications such as encephalitis, pneumonia, or disseminated disease. Traditional diagnostic methods, including viral culture and serology, often present limitations such as prolonged turnaround times, suboptimal sensitivity, or inability to distinguish between active infection and past exposure, making rapid clinical decisions challenging. The advancements in pcr varicella zoster virus detection have revolutionized this field, providing a robust solution to these diagnostic hurdles.
The global market for molecular diagnostics, especially within infectious disease testing, continues to exhibit rapid expansion. This growth is predominantly fueled by continuous technological innovations, the increasing global burden of infectious diseases, and a heightened emphasis on early and precise diagnosis to enhance patient outcomes and mitigate healthcare expenditures. Polymerase Chain Reaction (PCR) technology remains at the forefront of this diagnostic evolution, offering an unparalleled combination of sensitivity and specificity for direct detection of viral nucleic acids from diverse clinical samples. This paradigm shift underscores the critical importance of advanced diagnostic tools in contemporary healthcare, positioning highly specialized solutions like the Cowingene Varicella-Zoster Virus (VZV) Detection Kit (NATBox) as indispensable for clinical laboratories and research institutions worldwide.
Cowingene NATBox: Precision in PCR Varicella Zoster Virus Detection
The Cowingene Varicella-Zoster Virus (VZV) Detection Kit (NATBox) represents a cutting-edge molecular diagnostic solution for VZV. Leveraging the power of Nucleic Acid Testing (NAT) principles, this kit offers a highly robust and reliable methodology for the qualitative and quantitative detection of VZV DNA in various clinical specimens. The cornerstone of its operational excellence is Real-Time PCR (qPCR), a sophisticated technique renowned for its superior analytical sensitivity, quantitative capabilities, and rapid execution. This not only facilitates the precise identification of VZV but also allows for the quantification of viral load, which is often invaluable for monitoring disease progression, assessing treatment efficacy, and guiding clinical decisions in complex cases.
The development and manufacturing of the NATBox kit adhere to the most stringent international quality management systems, including ISO 13485 for medical devices. Each individual component – from highly specific primers and fluorescent probes to optimized reaction buffers and enzymes – undergoes rigorous validation and quality control assessments. This meticulous approach ensures unparalleled performance, batch-to-batch consistency, and extended shelf-life under recommended storage conditions. The kit's architecture is designed for ease of use and seamless integration into existing laboratory workflows, making it highly adaptable for a broad spectrum of clinical settings. This dedication to quality and user experience reflects Cowingene's unwavering commitment to delivering advanced, reliable, and high-performance diagnostic solutions for pcr varicella zoster virus testing.
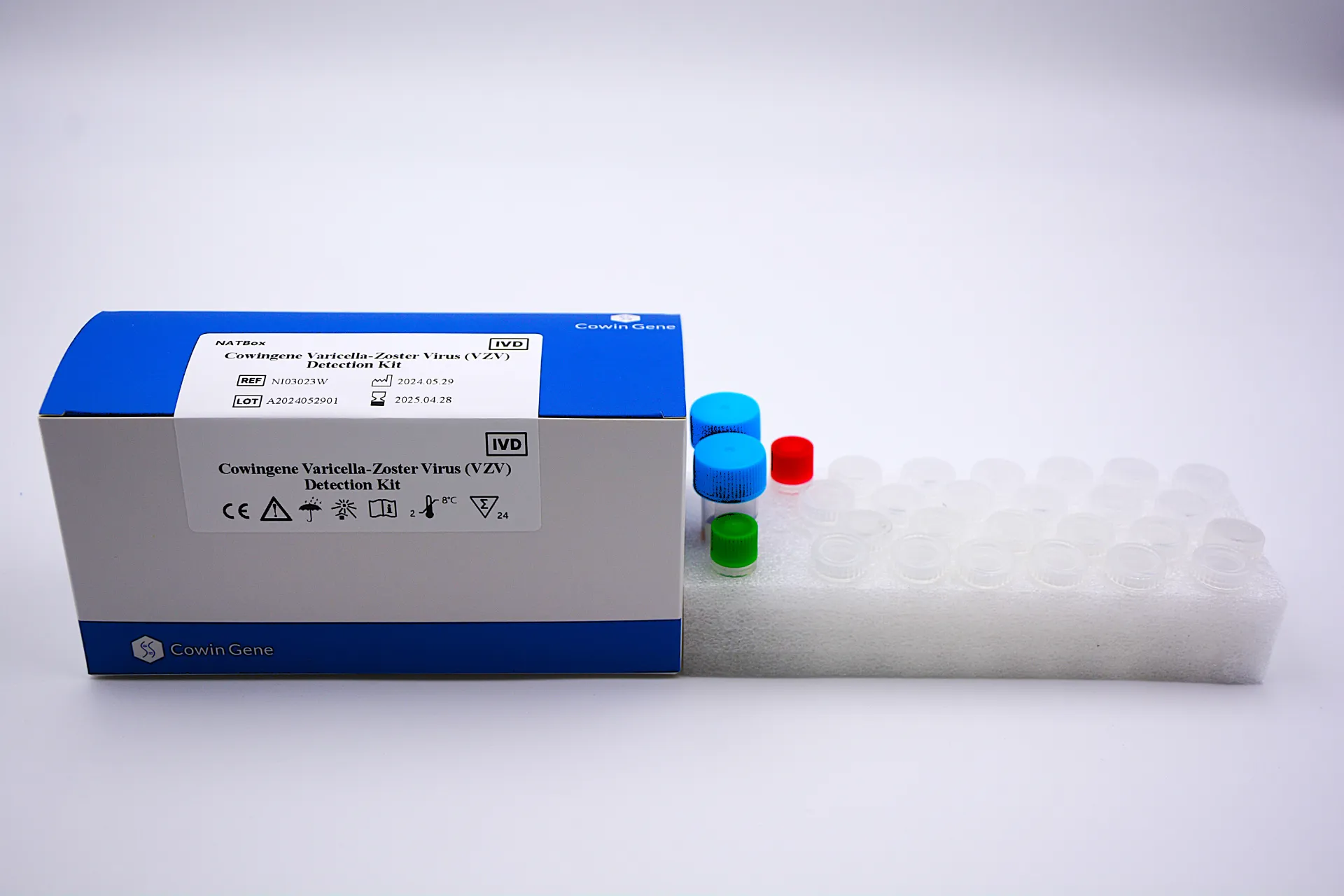
Figure 1: Cowingene VZV Detection Kit components, designed for efficient workflow integration.
Detailed Detection Process Flow:
- Sample Collection & Preparation: The diagnostic journey commences with the appropriate collection of clinical samples, which may include cerebrospinal fluid (CSF), plasma, whole blood, lesion swabs (from vesicles, crusts), or tissue biopsies. Proper handling, storage, and transport of samples are critical to preserve nucleic acid integrity and ensure accurate diagnostic outcomes.
- Nucleic Acid Extraction: VZV DNA is then isolated and purified from the collected samples. The Cowingene kit is engineered for compatibility with a wide array of commercially available automated and manual nucleic acid extraction systems, guaranteeing high purity and optimal yield of the target DNA, which is essential for subsequent amplification.
- Reaction Setup: Following extraction, the purified VZV DNA is combined with the kit’s proprietary master mix. This master mix contains all necessary reagents, including specific primers and highly sensitive fluorescent probes meticulously designed to target unique and highly conserved genomic regions of the VZV. The inclusion of validated internal controls ensures robust monitoring of extraction efficiency and inhibition, providing an additional layer of reliability.
- Real-Time PCR Amplification and Detection: The prepared reaction mixtures are subsequently loaded into a compatible real-time PCR thermocycler. During the precise thermal cycling protocol, if VZV DNA is present in the sample, it undergoes exponential amplification. Concurrently, the fluorescent signal generated by the specific probes is detected and monitored in real time, allowing for both qualitative detection and quantitative analysis of viral load.
- Data Analysis & Interpretation: Integrated software associated with the qPCR instrument performs sophisticated analysis of the amplification curves and cycle threshold (Ct) values. A positive result unequivocally indicates the presence of VZV DNA, while the Ct value provides a semi-quantitative measure of viral load, aiding clinicians in disease monitoring and therapeutic management.
- Quality Assurance & Validation: Every stage of the detection process, from reagent formulation to final data interpretation, is subjected to rigorous quality control checks. The kit incorporates both positive and negative controls to validate the entire workflow, ensuring the unwavering accuracy and reliability of every test result. This adherence to stringent quality standards underscores the kit's crucial role in critical diagnostic settings for pcr varicella zoster virus detection.
Technical Parameters & Performance of Cowingene VZV Detection Kit
For laboratories assessing new diagnostic platforms, a thorough understanding of precise technical parameters is paramount. The Cowingene Varicella-Zoster Virus (VZV) Detection Kit (NATBox) is meticulously engineered to deliver exceptional performance, consistently meeting and often exceeding the stringent demands of modern clinical diagnostics for pcr vzv. Its design prioritizes paramount analytical sensitivity and specificity, ensures rapid turnaround times, and offers broad compatibility with standard laboratory equipment. These inherent attributes collectively guarantee that diagnostic results are not only highly accurate but also rapidly accessible, which is critically important for acute VZV infections where timely therapeutic intervention can significantly mitigate severe clinical outcomes and improve patient prognosis.
| Parameter | Cowingene VZV Detection Kit (NATBox) | Industry Standard/Typical PCR Kit |
|---|---|---|
| Detection Target | VZV DNA (specific conserved gene regions) | Specific Viral DNA/RNA |
| Assay Type | Real-Time Quantitative PCR (qPCR) | qPCR or End-Point PCR |
| Limit of Detection (LoD) | ≤ 500 copies/mL (validated for various samples) | Typically 100-1000 copies/mL |
| Analytical Sensitivity | >98% (demonstrated in clinical evaluations) | Typically >90% |
| Analytical Specificity | 100% (no cross-reactivity with common herpesviruses, respiratory pathogens) | Typically >98% |
| Compatible Sample Types | CSF, Plasma, Whole Blood, Vesicle Fluid, Swabs (oral, nasopharyngeal), Tissue | Varied, depends on target pathogen |
| Reaction Time (PCR step) | Approx. 60-75 minutes | 60-120 minutes |
| Total Workflow Time (incl. extraction) | Approx. 90-120 minutes | 1.5 - 4 hours |
| Shelf Life | 12 months from manufacture (at -20°C) | 6-18 months |
| Compatible Instruments | Most mainstream real-time PCR platforms (e.g., Applied Biosystems, Bio-Rad, Roche LightCycler, Agilent) | Platform dependent |
These detailed specifications underscore the kit's robust and superior performance, positioning it as an ideal choice for laboratories that prioritize accuracy, efficiency, and broad utility. The exceptionally low Limit of Detection (LoD) empowers the kit to detect even minute quantities of viral DNA, which is critically important for early diagnosis, managing latent infections, and identifying atypical disease presentations. Furthermore, its demonstrated 100% analytical specificity virtually eliminates the risk of false positives due to cross-reactivity with other common pathogens, providing clinicians with highly reliable diagnostic information. This reliability is paramount for guiding optimal patient care and implementing effective public health measures, especially concerning advanced varicella zoster virus dna pcr detection.
Diverse Applications & Strategic Advantages
The extensive versatility of the Cowingene VZV Detection Kit (NATBox) allows its deployment across numerous critical application scenarios within diverse healthcare and research settings. Its high-performance characteristics render it indispensable in environments where rapid and precise VZV diagnosis directly influences clinical decisions and significantly impacts patient outcomes. Predominantly, the kit serves hospital diagnostic laboratories, enabling swift and accurate identification of VZV in patients presenting with suspicious symptoms. This capability facilitates prompt initiation of targeted antiviral therapy, crucial for preventing severe disease progression, and aids in implementing effective infection control measures to prevent nosocomial transmission within healthcare facilities. It also plays a vital role in public health surveillance, contributing significantly to a deeper understanding of VZV epidemiology and supporting effective outbreak response efforts.
Beyond routine clinical diagnostics, the Cowingene VZV Detection Kit finds extensive application in specialized clinical areas, such as ophthalmology for diagnosing ocular herpes zoster, and neurology for investigating VZV-associated neurological syndromes including VZV encephalitis, myelitis, or vasculopathy. Research institutions also heavily rely on its robust performance for conducting studies on VZV latency, mechanisms of viral reactivation, assessment of antiviral drug resistance, and evaluating the efficacy of VZV vaccines. The strategic technical advantages of utilizing a pcr varicella zoster virus kit like NATBox are profound: significantly reduced turnaround times compared to conventional diagnostic methodologies, superior analytical sensitivity for detecting even extremely low viral loads, and exceptionally high specificity that minimizes the potential for false positive results. This translates directly into optimized patient management, substantial reductions in healthcare costs by enabling targeted and effective treatment, and overall enhancement of public health safety and preparedness against VZV.

Figure 2: Streamlined laboratory workflow enabled by the Cowingene NATBox kit's intuitive design.
Advantages in Application:
- Rapid Diagnostics: Enables clinicians to make faster and more informed treatment decisions, which is particularly crucial for immunocompromised patients or those presenting with severe, life-threatening manifestations of VZV infection.
- Early Detection: Facilitates the detection of viral DNA even during the prodromal phase or before the full development of antibody responses or overt clinical symptoms, allowing for proactive and preventative intervention strategies.
- High Specificity: Minimizes the occurrence of false positives, thereby preventing unnecessary treatments, reducing patient anxiety, and optimizing resource allocation within healthcare systems.
- Quantitative Analysis: Provides valuable viral load data, which is essential for monitoring disease progression, assessing the effectiveness of antiviral therapies, and guiding decisions on treatment duration and dosage.
- Broad Versatility: Compatible with a diverse array of clinical sample types and a wide spectrum of common qPCR platforms, offering exceptional flexibility and adaptability to meet the varied operational needs of different laboratories.
- Streamlined Workflow: Designed for maximum efficiency, minimizing hands-on time, reducing the potential for human error, and increasing overall laboratory throughput for varicella zoster virus dna pcr testing.
Industry Positioning and Customized Solutions
Within the intensely competitive landscape of molecular diagnostics, Cowingene consistently distinguishes itself through an unwavering commitment to pioneering innovation, uncompromising quality, and unparalleled comprehensive customer support. While numerous manufacturers offer PCR-based diagnostic kits for infectious diseases, Cowingene’s deliberate focus on robust performance, intuitive ease of use, and profound adaptability strategically positions the NATBox kit as a superior and preferred choice for laboratories demanding the most reliable pcr vzv detection capabilities. Our diagnostic kits are meticulously developed with a deep, nuanced understanding of evolving clinical needs, rigorously ensuring that they not only meet but frequently surpass established industry benchmarks for analytical sensitivity and specificity.
Cowingene is uniquely equipped to offer highly flexible and customized solutions, meticulously tailored to address the diverse and often specific requirements of individual laboratories, large hospital networks, and integrated healthcare systems. Recognizing that each client may possess distinct operational parameters and logistical demands, we provide adaptable packaging sizes, advantageous bulk supply options, and dedicated technical assistance for seamless integration into existing automated laboratory systems. Our highly responsive and expert technical support team ensures effortless implementation and sustained operational efficiency, thereby fostering enduring partnerships built upon a foundation of mutual trust and shared success. This bespoke approach, harmoniously coupled with our rigorous multi-tiered quality assurance processes, unequivocally solidifies Cowingene’s esteemed reputation as a reliable, innovative, and forward-thinking partner in the forefront of molecular diagnostics.
Figure 3: Cowingene VZV kit utilized in a laboratory setting, demonstrating seamless integration.
Key Aspects of Trustworthiness:
- Certifications and Compliance: All Cowingene products, including the NATBox kit, are diligently manufactured under stringent quality management systems fully compliant with leading international standards such as ISO 13485, ensuring the utmost level of product quality, safety, and regulatory adherence.
- Transparent Delivery Cycles: We maintain clear, proactive communication regarding all aspects of order processing and delivery timelines. Our logistics are optimized for efficiency, ensuring products reach our valued clients promptly, typically within 5-10 business days for standard orders, varying based on geographical location and shipping method.
- Comprehensive Warranty and Dedicated Support: Every Cowingene kit is accompanied by a standard warranty meticulously covering manufacturing defects. Furthermore, our highly responsive and knowledgeable customer support team is readily available to provide expert assistance with technical queries, troubleshooting, and protocol optimization, guaranteeing a consistently smooth and successful user experience for pcr varicella zoster virus testing.
- Validated Performance Data: Extensive internal validation studies, complemented by independent external performance evaluations and collaborative research, consistently confirm the exceptional reliability, accuracy, and reproducibility of our pcr varicella zoster virus detection solutions.
Frequently Asked Questions (FAQ)
Q1: What types of samples are compatible with the Cowingene VZV Detection Kit?
The Cowingene Varicella-Zoster Virus (VZV) Detection Kit is engineered for exceptional versatility and is compatible with a comprehensive range of clinical sample types. These include, but are not limited to, cerebrospinal fluid (CSF), plasma, whole blood, lesion swabs (collected from vesicles or crusted lesions), nasopharyngeal swabs, and various tissue biopsies. This broad compatibility ensures that clinicians and researchers can effectively utilize the kit for accurately diagnosing VZV infections across diverse patient presentations and disease manifestations, offering significant flexibility in sample collection based on specific clinical needs.
Q2: How long does it typically take to obtain results using this pcr vzv kit?
The total turnaround time for obtaining definitive results using the Cowingene VZV Detection Kit (NATBox) is remarkably rapid, typically ranging from approximately 90 to 120 minutes. This efficient timeline encompasses both the nucleic acid extraction process from the collected sample and the subsequent real-time PCR amplification and detection steps. Such swift turnaround is a substantial advantage, particularly in acute clinical environments where prompt and accurate diagnosis of varicella zoster virus dna pcr is critical for initiating timely antiviral therapy, guiding patient management, and preventing further transmission. It drastically reduces the diagnostic waiting period compared to conventional culture-based methods.
Q3: Is the Cowingene VZV Detection Kit compatible with existing automated laboratory systems?
Yes, the Cowingene VZV Detection Kit (NATBox) is thoughtfully designed to ensure seamless compatibility with most common automated nucleic acid extraction systems and a wide spectrum of real-time PCR instruments that are routinely utilized in modern diagnostic laboratories today. This inherent compatibility greatly facilitates its integration into existing high-throughput laboratory workflows, significantly reducing manual hands-on time, minimizing the potential for human error, and notably increasing overall laboratory throughput. For specific automation platforms, our dedicated technical support team is readily available to provide expert guidance and assist with protocol optimization, ensuring efficient and reliably accurate performance.
Conclusion: Advancing VZV Diagnostics with Precision
The imperative for accurate, rapid, and consistently reliable diagnosis of Varicella-Zoster Virus infections cannot be overemphasized, particularly across diverse clinical scenarios ranging from routine diagnostics to critical care interventions and epidemiological surveillance initiatives. The Cowingene Varicella-Zoster Virus (VZV) Detection Kit (NATBox) stands as a definitive testament to the remarkable advancements in molecular diagnostics, offering a highly sensitive and specific pcr varicella zoster virus solution. By meticulously integrating cutting-edge pcr vzv technology with rigorous quality control protocols and comprehensive customer support, Cowingene empowers laboratories with an indispensable tool for significantly enhancing patient outcomes and streamlining complex diagnostic workflows. Our unwavering commitment to continuous innovation and diagnostic excellence ensures that healthcare providers universally have access to the most effective and reliable means of detecting and managing VZV infections, thereby empowering informed clinical decisions and robustly improving global public health.
References
- Mahalingam, R., & Wellish, M. (2018). Varicella-Zoster Virus. In: Scheld, W.M., Whitley, R.J., Marra, C.M. (eds) Infections of the Central Nervous System. Lippincott Williams & Wilkins.
- Pichichero, M.E. (2004). Varicella-Zoster Virus Infection. In: Clinical Practice of Pediatric Infectious Diseases. Elsevier.
- Centers for Disease Control and Prevention. (2023). Varicella (Chickenpox) and Herpes Zoster (Shingles): Clinical Information.
- European Centre for Disease Prevention and Control. (2021). Factsheet for health professionals on Varicella-Zoster virus.
- Steiner, I., Kennedy, P. G. E., & Nath, A. (2014). The neurotropic herpesviruses: herpes simplex and varicella-zoster. Lancet Neurology, 13(10), 1010-1021.
Related PRODUCTS
-
Fast & Accurate Dengue PCR Test for Early Diagnosis
NewsAug.21,2025 -
Accurate Legionella Pneumophila Detection PCR Kits
NewsAug.19,2025 -
Rapid & Accurate PCR Influenza A Testing Solutions
NewsAug.18,2025 -
Accurate HPV Genotyping Test Kit | Home Self-Sampling
NewsAug.17,2025 -
Accurate Dengue PCR Test | Rapid RNA & RT-PCR Detection
NewsAug.15,2025 -
Advanced Respiratory Panel Lab Test for Quick Diagnosis
NewsAug.14,2025







