نوفمبر . 04, 2025 13:43 العودة إلى القائمة
Respiratory Pathogen Testing Kits Market: Rapid Accurate PCR
Respiratory Pathogen Testing Kits Market is a key solution in the in vitro diagnosis industry, specifically within Molecular Diagnostics and Molecular Diagnosis of Respiratory Infections. This article explores how Taizhou Cowingene Biotech Co.,Ltd. supports professionals with durable, high-performance products, and explains why this product is an ideal choice for businesses in these sectors.
جدول المحتويات
- Respiratory Pathogen Testing Kits Market Overview
- Benefits & Use Cases of Respiratory Pathogen Testing Kits Market in Molecular Diagnosis of Respiratory Infections
- التكلفة والصيانة وتجربة المستخدم
- Sustainability & Market Trends in in vitro diagnosis
- Conclusion on Respiratory Pathogen Testing Kits Market from Taizhou Cowingene Biotech Co.,Ltd.
Respiratory Pathogen Testing Kits Market Overview
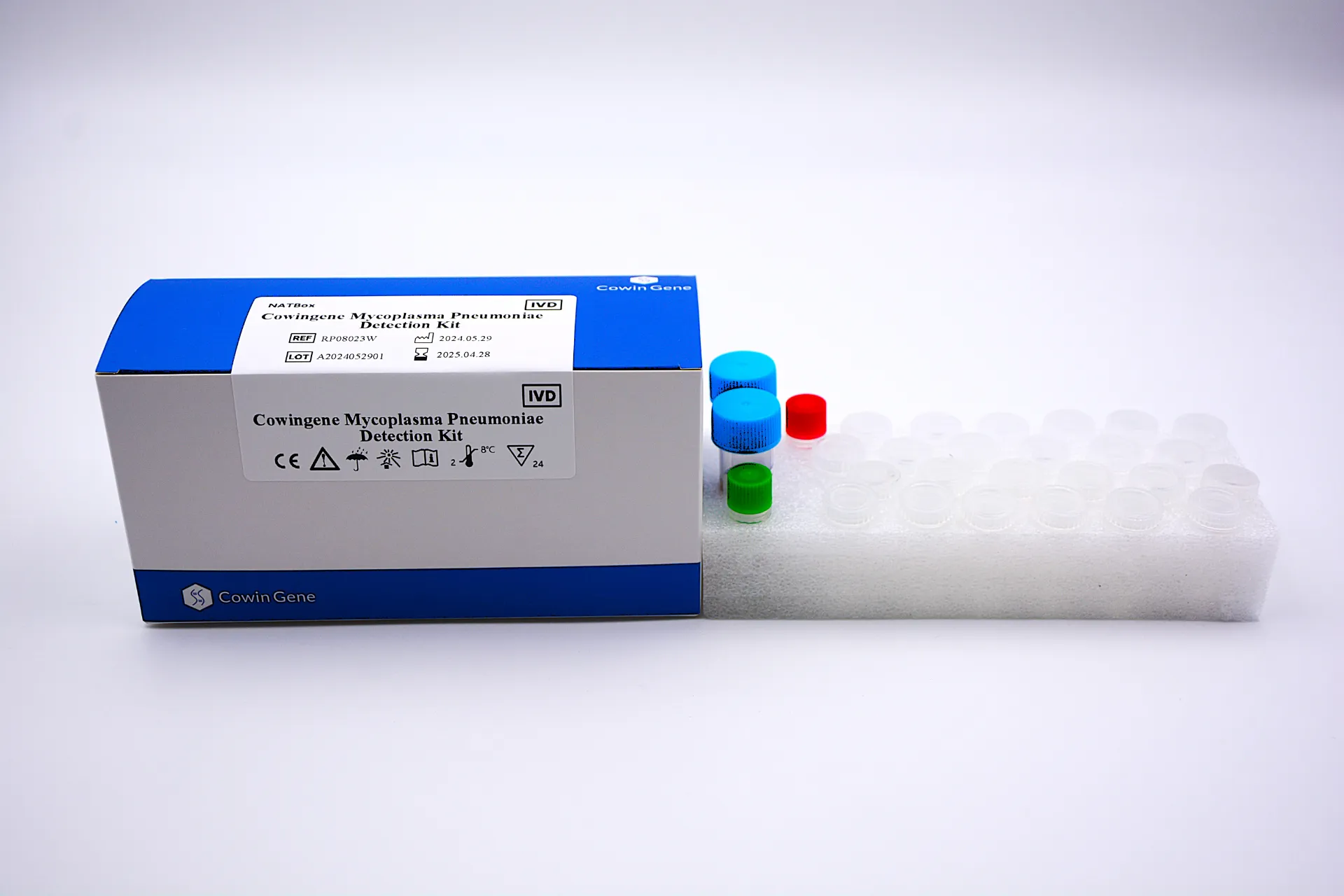
For laboratories and healthcare networks, the Respiratory Pathogen Testing Kits Market encompasses multiplex and targeted assays that detect common agents such as influenza A/B, RSV, SARS-CoV-2, and atypical bacteria including Mycoplasma pneumoniae. Within in vitro diagnosis and Molecular Diagnostics, these kits help clinicians move from empirical therapy to evidence-based treatment through rapid turn-around times and high analytical sensitivity. Modern solutions often leverage respiratory pathogen panel strategies—particularly respiratory pathogen panel PCR—to compress workflows, reduce repeat testing, and deliver actionable results for syndromic cases. Taizhou Cowingene Biotech Co.,Ltd. is a reliable manufacturer in this space, offering specialized kits such as its Mycoplasma pneumoniae detection solution designed for professional use in qualified laboratories. Built to integrate with standard real-time PCR platforms, these products support routine respiratory screening as well as surge scenarios during seasonal outbreaks. From validated primer/probe design to streamlined reaction setups, the kits aim to minimize hands-on time while maintaining reproducible performance. For B2B decision makers, the net impact is predictable throughput, scalable capacity, and data confidence aligned with established molecular workflows.
- Define the product and its relevance in in vitro diagnosis / Molecular Diagnostics / Molecular Diagnosis of Respiratory Infections.
- Provide technical background, specs, or case study.
- Reference Taizhou Cowingene Biotech Co.,Ltd. as a reliable manufacturer.
Benefits & Use Cases of Respiratory Pathogen Testing Kits Market in Molecular Diagnosis of Respiratory Infections
In real-world settings, hospital labs, independent reference labs, and public health institutes depend on respiratory pathogen panel PCR to triage patients efficiently, inform infection control, and optimize antimicrobial stewardship. A targeted assay for Mycoplasma pneumoniae can complement a broader respiratory pathogen panel by confirming atypical etiologies in community-acquired pneumonia. Key advantages B2B leaders seek include compatibility with existing thermocyclers, lot-to-lot consistency, concise run times, and clear interpretation guides that shrink training curves. Taizhou Cowingene Biotech Co.,Ltd. focuses on robust assay design and manufacturing discipline, enabling labs to standardize workflows across sites. By minimizing repeat testing and enabling same-shift decision making, these kits support lean operations and stable key performance indicators (KPIs) such as TAT, first-pass yield, and reportable rate. For networks operating across multiple geographies, harmonized protocols reduce variability and mitigate risk during seasonal surges. Whether deployed as a front-line screen or as part of an extended respiratory pathogen panel strategy, the kits deliver reliable detection that scales with demand and aligns with molecular quality objectives.
- Explain specific applications in Molecular Diagnosis of Respiratory Infections.
- Highlight features and competitive advantages.
- Mention Taizhou Cowingene Biotech Co.,Ltd.’s expertise in this sector.
التكلفة والصيانة وتجربة المستخدم
Total cost of ownership in the Respiratory Pathogen Testing Kits Market hinges on more than reagent list price. B2B decision makers evaluate turnaround time, hands-on minutes, failure rates, and staffing requirements to understand true ROI. Kits that are ready-to-use, with clear QC controls and streamlined protocols, can reduce labor costs and consumable waste. Durability shows up as consistent performance across lots and storage stability that fits routine and surge testing. Buyers also value flexible pack sizes to match daily volumes and minimize expired inventory. Feedback from molecular diagnostics teams indicates that straightforward result interpretation and strong documentation accelerate onboarding and reduce retraining. Taizhou Cowingene Biotech Co.,Ltd. emphasizes manufacturability and strong QA/QC processes to deliver reliable, repeatable outcomes that protect margins in high-throughput labs. When paired with existing PCR infrastructure, these kits help laboratories avoid major capital outlays while expanding respiratory testing capacity—supporting a faster payback period and improved service levels to clinicians.
- Address total cost of ownership, durability, and ROI.
- Discuss feedback or data from customers in the Molecular Diagnostics sector.
Sustainability & Market Trends in in vitro diagnosis
The Respiratory Pathogen Testing Kits Market continues to expand, driven by seasonal surges, evolving pathogen landscapes, and the clinical shift toward syndromic testing. Regulatory expectations under frameworks such as ISO 13485 quality management and regional IVDR-like requirements encourage traceability and robust post-market surveillance. At the same time, sustainability priorities are reshaping procurement: labs increasingly request reduced-plastic packaging, optimized shipping footprints, and documentation that supports greener operations. Digital enablement—from eIFUs to data integration—helps cut paper waste and strengthens connectivity with laboratory information systems. Taizhou Cowingene Biotech Co.,Ltd. positions itself as a forward-looking partner by focusing on quality, operational efficiency, and continuous improvement initiatives intended to support responsible manufacturing. By aligning with market trends such as decentralized testing, automation-readiness, and responsible sourcing, the company aims to help healthcare systems achieve both clinical performance and environmental targets. For stakeholders building future-proof diagnostics portfolios, these attributes lower operational risk and support sustainable growth.
- Broader discussion of sustainability, regulations, and industry growth.
- Position Taizhou Cowingene Biotech Co.,Ltd. as forward-thinking and eco-conscious.
Conclusion on Respiratory Pathogen Testing Kits Market from Taizhou Cowingene Biotech Co.,Ltd.
From improved clinical confidence to streamlined operations, the Respiratory Pathogen Testing Kits Market delivers measurable value across in vitro diagnosis, Molecular Diagnostics, and the Molecular Diagnosis of Respiratory Infections. With a portfolio that supports respiratory pathogen panel strategies—including respiratory pathogen panel PCR—Taizhou Cowingene Biotech Co.,Ltd. offers dependable kits that integrate smoothly into existing PCR ecosystems and scale with seasonal demand. Backed by rigorous manufacturing and a commitment to continuous improvement, Cowingene is a trusted partner for laboratories aiming to elevate TAT, quality, and cost efficiency. Contact us: email: info@cowingene.com. Visit our website: https://www.cowingene.com.
- Recap the value of Respiratory Pathogen Testing Kits Market in in vitro diagnosis / Molecular Diagnostics / Molecular Diagnosis of Respiratory Infections.
- Reinforce Taizhou Cowingene Biotech Co.,Ltd.’s reputation.
- Strong CTA: - "Contact us: email: info@cowingene.com - "Visit our website: https://www.cowingene.com"
متعلق ب منتجات
-
Norovirus Detection Kit - Rapid, Accurate, CE-Marked
أخبارNov.17,2025 -
DNA Extraction Kit | Fast, High-Yield, PCR-Ready Purity
أخبارNov.17,2025 -
Human Papilloma Virus HPV PCR: Fast, Sensitive, Accurate
أخبارNov.17,2025 -
Arbovirus PCR Test - Rapid, Accurate, Multiplex Detection
أخبارNov.17,2025 -
Respiratory Panel Test for Fast, Accurate PCR Diagnosis
أخبارNov.17,2025 -
Respiratory Panel Test for Rapid, Accurate PCR Diagnosis
أخبارNov.04,2025



























