Agu . 12, 2025 04:00 Back to list
Rapid RSV Detection Kit: Accurate & Reliable Testing
The Growing Imperative of RSV Detection: Trends and Innovations
Respiratory Syncytial Virus (RSV) poses a significant global health challenge, particularly for infants, young children, the elderly, and immunocompromised individuals. Its clinical manifestations often overlap with influenza and other respiratory pathogens, making accurate and rapid diagnosis critical for effective patient management and public health interventions. The demand for advanced diagnostic solutions, including comprehensive RSV detection kit, is steadily escalating. Industry trends point towards a strong emphasis on multiplex capabilities, enabling simultaneous detection of multiple pathogens like Influenza A/B and RSV, alongside improved sensitivity, specificity, and turnaround time. This convergence enhances diagnostic efficiency, reduces resource strain, and facilitates timely treatment decisions, ultimately improving patient outcomes during peak respiratory illness seasons.
Recent epidemiological data underscore the burden of RSV. According to the Centers for Disease Control and Prevention (CDC), RSV causes approximately 58,000 to 80,000 hospitalizations in children younger than 5 years old and 6,000 to 10,000 deaths among adults aged 65 and older in the United States each year. The global market for RSV diagnostics is projected to grow significantly, driven by rising awareness, increasing incidence of respiratory infections, and advancements in molecular diagnostics. Innovations in lyophilization and point-of-care (POC) testing are key drivers, offering enhanced stability, simplified workflows, and accessibility. The strategic integration of robust and reliable RSV kit solutions into routine clinical practice is paramount for mitigating the impact of seasonal outbreaks and supporting comprehensive respiratory disease surveillance.
Introducing the Cowingene Influenza A/B and RSV Detection Kit (Lyophilized)
At the forefront of advanced diagnostics, the Cowingene Influenza A/B and RSV Detection Kit (Lyophilized) stands as a testament to precision and convenience in molecular diagnostics. This state-of-the-art RSV detection kit is engineered for the qualitative detection of nucleic acid from Influenza A virus, Influenza B virus, and Respiratory Syncytial Virus (RSV) in human respiratory tract samples, including nasopharyngeal swabs, oropharyngeal swabs, and sputum. Its lyophilized format represents a significant technological leap, offering unparalleled stability and ease of use, eliminating the need for cold chain transportation and storage for most of its shelf life. This reduces logistical complexities and costs, making it an ideal solution for diverse clinical settings, from urban hospitals to remote laboratories.
The design of the Cowingene kit leverages highly specific primers and probes for each target, ensuring minimal cross-reactivity and maximizing diagnostic accuracy. The multiplex capability allows for a comprehensive respiratory panel from a single patient sample, optimizing laboratory workflow and providing clinicians with crucial information for differential diagnosis more rapidly. The lyophilized format simplifies reagent preparation, reducing the risk of pipetting errors and accelerating the overall testing process. This kit embodies Cowingene’s commitment to delivering reliable, efficient, and user-friendly diagnostic tools that meet the rigorous demands of modern healthcare, significantly enhancing the capability for swift and accurate RSV detection and co-infection identification.
Unpacking the Technology: The Precision Manufacturing Process of RSV Detection Kits
The manufacturing of a high-quality RSV detection kit like the Cowingene Influenza A/B and RSV Detection Kit involves a sophisticated, multi-stage process designed to ensure unparalleled accuracy, stability, and reliability. Unlike traditional manufacturing of heavy machinery, where processes like casting and forging define structural integrity, the production of molecular diagnostic kits focuses on precise biochemical synthesis, stringent quality control, and advanced formulation techniques. The journey begins with the meticulous synthesis and purification of highly specific oligonucleotide primers and fluorescent probes designed to target unique genomic regions of Influenza A, Influenza B, and RSV. These critical components are synthesized under controlled cleanroom environments to prevent contamination and ensure batch-to-batch consistency.
Following synthesis, these reagents, along with high-fidelity enzymes and reaction buffers, undergo rigorous quality checks for purity, concentration, and functional performance. A crucial step for the Cowingene kit is the lyophilization process. This involves freezing the pre-mixed reaction components and then sublimating the ice under vacuum, transforming the liquid mixture into a stable, porous solid. This advanced manufacturing technique significantly extends the product’s shelf life, typically up to 12 months from the date of manufacture, and eliminates the need for cold chain shipping, reducing logistical complexities and environmental impact. The lyophilized pellets are then precisely dispensed into reaction tubes or plates, which are then sealed in moisture-protective packaging.
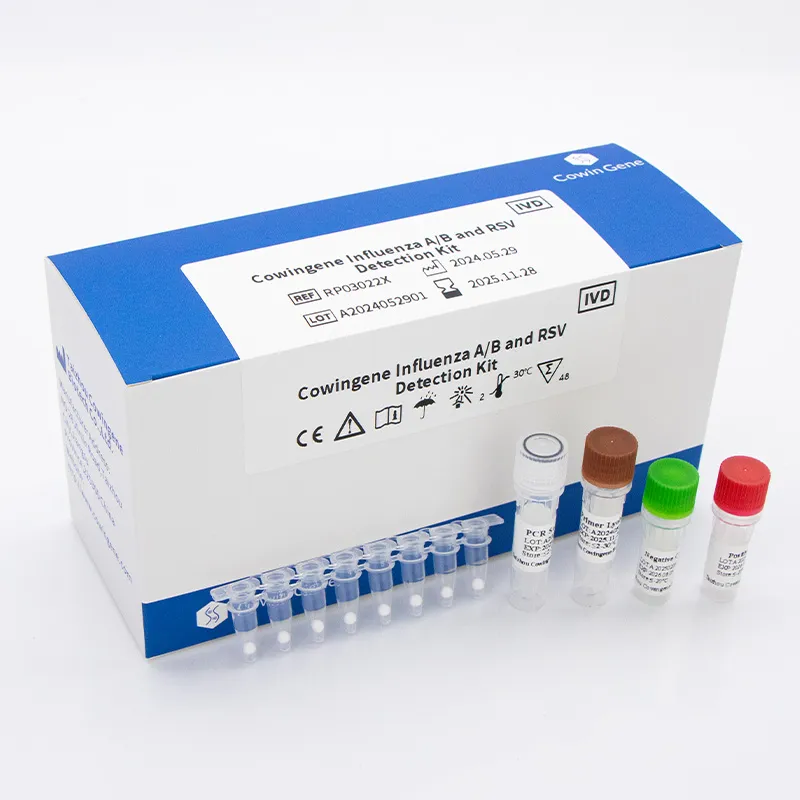
Throughout the entire manufacturing journey, adherence to stringent international standards is paramount. Cowingene's processes comply with ISO 13485:2016 for medical devices quality management systems, and the kit holds CE-IVD marking, signifying conformity with European Union health, safety, and environmental protection standards. Each batch undergoes comprehensive final quality assurance, including sensitivity, specificity, and stability testing, mirroring clinical performance. This rigorous protocol ensures that every RSV kit delivered meets the highest benchmarks for diagnostic accuracy and user convenience, ready for immediate use in laboratories worldwide, supporting critical sectors such as clinical diagnostics, public health surveillance, and research institutions.
Technical Specifications & Performance Data: Benchmarking Diagnostic Excellence
The efficacy of an RSV detection kit is defined by its core technical specifications and validated performance data. The Cowingene Influenza A/B and RSV Detection Kit (Lyophilized) is characterized by exceptional analytical sensitivity, clinical specificity, and rapid turnaround time, making it a robust tool for high-volume diagnostic laboratories. Key performance indicators are meticulously evaluated during product development and ongoing quality control, ensuring consistent and reliable results.
| Parameter | Cowingene Influenza A/B & RSV Detection Kit (Lyophilized) | Typical Industry Benchmarks (PCR-based) |
|---|---|---|
| Detection Targets | Influenza A, Influenza B, RSV | Single or Multiplex (e.g., Flu A/B, RSV, CoV) |
| Sample Types | Nasopharyngeal swabs, Oropharyngeal swabs, Sputum | Nasopharyngeal swabs, washes, aspirates, sputum |
| Limit of Detection (LoD) | Flu A: < 200 copies/mL Flu B: < 200 copies/mL RSV: < 250 copies/mL |
150-1000 copies/mL (varies by target) |
| Clinical Sensitivity | Flu A: > 97.5% Flu B: > 97.0% RSV: > 96.8% |
90-98% |
| Clinical Specificity | Flu A: > 99.0% Flu B: > 99.0% RSV: > 99.0% |
95-100% |
| Reaction Time | Approx. 60 minutes (after RNA extraction) | 45-90 minutes (PCR-based) |
| Storage Conditions | 2-30°C (lyophilized format) | -20°C (liquid reagents) |
| Shelf Life | 12 months | 9-18 months |
The robust analytical performance, characterized by a low Limit of Detection (LoD), ensures early and accurate identification of infections, even with low viral loads. The high clinical sensitivity and specificity minimize false negatives and false positives, providing clinicians with confidence in their diagnostic decisions. Furthermore, the lyophilized format significantly simplifies logistics and storage, making it an ideal choice for facilities seeking to optimize their operational efficiency and environmental footprint. This comprehensive performance profile underscores the kit's capability to deliver reliable and actionable results for effective RSV detection and management.
Application Scenarios & Real-World Impact: Enhancing Clinical Workflow
The Cowingene Influenza A/B and RSV Detection Kit (Lyophilized) is designed for versatile application across a spectrum of healthcare settings, significantly enhancing diagnostic capabilities and patient care. Its robust performance and convenient format make it indispensable in various scenarios where rapid and accurate respiratory virus identification is critical.
- ✓ Hospital Laboratories & Clinical Diagnostics: In acute care settings, timely RSV detection is crucial for patient triage, isolation protocols, and initiating appropriate antiviral therapies. This kit enables rapid differentiation between Flu A/B and RSV, guiding specific treatment pathways and preventing unnecessary antibiotic use. Its high throughput potential supports busy hospital labs during peak seasons.
- ✓ Public Health Surveillance: For epidemiologists and public health agencies, consistent and accurate data on circulating respiratory viruses are vital for monitoring trends, predicting outbreaks, and implementing public health interventions. The kit provides reliable data, contributing to robust surveillance programs that inform vaccination strategies and resource allocation.
- ✓ Pediatric and Geriatric Care: These vulnerable populations are disproportionately affected by RSV. Rapid diagnosis allows for prompt supportive care, reducing the risk of severe complications and hospitalizations. The kit's ease of use and stability make it suitable for a wide range of facilities serving these patient groups.
- ✓ Emergency Departments & Urgent Care Centers: Fast results are paramount in emergency settings. The efficiency of this rsv kit allows clinicians to quickly identify the causative agent of respiratory symptoms, enabling faster patient flow, reducing wait times, and improving overall operational efficiency.
The real-world impact extends beyond mere diagnosis. By providing rapid and precise identification of respiratory pathogens, this rsv detection kit empowers healthcare providers to optimize patient management, implement timely infection control measures, and conserve valuable healthcare resources. Its adaptability to diverse clinical environments, combined with the logistical advantages of its lyophilized format, positions it as an essential tool in the ongoing global effort to combat respiratory infectious diseases and enhance public health preparedness.
Competitive Landscape & Cowingene's Strategic Advantage
The market for respiratory virus detection kits is highly competitive, populated by numerous manufacturers offering various technologies, including rapid antigen tests, molecular PCR assays, and immunoassay-based solutions. While each type of RSV detection kit serves specific clinical needs, molecular diagnostics, particularly real-time PCR, remains the gold standard for its unparalleled sensitivity and specificity. Cowingene distinguishes itself within this landscape through a combination of innovative technology, rigorous quality, and a commitment to user-centric design.
Cowingene's strategic advantages include:
- ● Lyophilized Format: This is a key differentiator. Unlike many liquid-based PCR kits that require continuous cold chain storage and transport, the lyophilized format of the Cowingene RSV kit offers superior stability at ambient temperatures (2-30°C). This significantly reduces shipping costs, eliminates the risk of reagent degradation due to temperature excursions, and simplifies inventory management for laboratories globally, including those in regions with limited cold chain infrastructure.
- ● Multiplex Capability: The ability to simultaneously detect Influenza A, Influenza B, and RSV from a single sample streamlines laboratory workflows, reduces reagent consumption, and provides a comprehensive diagnostic picture in a single run. This contrasts with single-target kits that require multiple tests for differential diagnosis.
- ● High Performance & Reliability: As demonstrated by its excellent sensitivity and specificity, the Cowingene RSV detection kit consistently delivers accurate results, minimizing false positives and negatives. This level of reliability is critical for clinical decision-making and public health surveillance.
- ● Ease of Use: The lyophilized master mix simplifies preparation, reducing pipetting steps and the potential for human error, which is a common challenge with multi-component liquid reagent systems. This contributes to faster hands-on time and improved laboratory efficiency.
By focusing on these core strengths, Cowingene not only meets but often exceeds the performance and logistical demands of modern diagnostic laboratories, providing a superior value proposition compared to many alternatives on the market for comprehensive respiratory virus detection.
Tailored Solutions & Unwavering Customer Support
Cowingene understands that every laboratory and healthcare system has unique requirements. While the core functionality of our RSV detection kit is standardized to ensure consistent quality, we are committed to providing flexible and responsive support to meet diverse operational needs. For large-scale procurement or specific integration requirements, Cowingene offers tailored solutions, including bulk packaging options, customized logistic plans, and dedicated technical assistance. Our goal is to ensure seamless adoption and optimal performance of our diagnostic products within your existing infrastructure.
Our dedication extends beyond product delivery to comprehensive customer support. This includes:
- ● Technical Training: We provide detailed training sessions for laboratory personnel, ensuring they are proficient in the use of the Cowingene Influenza A/B and RSV detection kit, from sample preparation to data interpretation.
- ● Dedicated Support Team: Our team of highly skilled technical specialists is available to address any queries, troubleshoot issues, and provide expert guidance, ensuring uninterrupted diagnostic workflow.
- ● Resource Materials: Access to comprehensive user manuals, protocols, FAQs, and scientific validation reports is readily available to support our clients' diagnostic operations.
Our commitment to long-term partnerships ensures that clients receive not just a product, but a complete solution backed by robust support, facilitating accurate RSV detection and overall laboratory success.
Trust & Assurance: FAQs, Delivery & Quality Commitment
Building trust is fundamental to Cowingene's mission. We stand behind the quality and performance of our Influenza A/B and RSV detection kit with transparent policies on quality assurance, delivery, and customer support. Our commitment is underpinned by adherence to global quality standards and a responsive support framework.
Frequently Asked Questions (FAQs)
-
Q: What is the primary advantage of a lyophilized RSV kit?
A: The lyophilized format offers superior stability, allowing storage at ambient temperatures (2-30°C) without the need for a cold chain. This reduces shipping costs, simplifies logistics, and ensures reagent integrity even in challenging environments. -
Q: Can this kit detect co-infections of Influenza and RSV?
A: Yes, the Cowingene kit is a multiplex assay designed for the simultaneous detection and differentiation of Influenza A, Influenza B, and RSV nucleic acids from a single sample, providing a comprehensive diagnostic profile. -
Q: What is the typical turnaround time for results using this RSV detection kit?
A: After nucleic acid extraction, the real-time PCR reaction typically completes within approximately 60 minutes, enabling rapid diagnosis and timely clinical decisions. -
Q: What regulatory certifications does the kit hold?
A: The Cowingene Influenza A/B and RSV Detection Kit is CE-IVD marked, signifying compliance with the essential requirements of the European In Vitro Diagnostic Medical Devices Regulation. Our manufacturing processes adhere to ISO 13485 standards.
Delivery & Quality Assurance
Delivery Cycle: Cowingene maintains efficient logistics to ensure timely delivery of our products worldwide. Standard lead times are communicated upon order confirmation, with expedited options available for urgent requirements. Our robust supply chain, facilitated by the lyophilized format, minimizes transit risks and delays associated with cold chain maintenance.
Warranty and Support: Each Cowingene RSV detection kit is manufactured under strict quality control, ensuring performance consistency. We offer a standard product warranty covering manufacturing defects and performance as per specifications. Our dedicated customer support team is available to assist with technical inquiries, troubleshooting, and provide comprehensive guidance, reinforcing our commitment to your diagnostic success.
Authoritative References
- Centers for Disease Control and Prevention. (2023). Respiratory Syncytial Virus (RSV) Information.
- World Health Organization. (2023). WHO guidelines for the clinical management of severe acute respiratory infection.
- National Institute of Allergy and Infectious Diseases (NIAID). (2022). Research on Respiratory Syncytial Virus (RSV).
- European Centre for Disease Prevention and Control (ECDC). (2023). Respiratory Syncytial Virus (RSV) data and surveillance.
Related PRODUCTS
-
Advanced Respiratory Panel Lab Test for Quick Diagnosis
NewsAug.14,2025 -
PCR Varicella Zoster Virus: Fast, Accurate Detection
NewsAug.13,2025 -
Respiratory Pathogen Panel Kits: Rapid & Accurate Testing
NewsAug.11,2025 -
Mycoplasma Pneumoniae Detection: Accurate PCR Tests
NewsAug.10,2025 -
Reliable Chlamydophila Pneumoniae PCR Test for Accurate Diagnosis
NewsAug.09,2025