Aug . 02, 2025 10:00 Back to list
High-Precision CMV DNA Quantitative PCR Testing | Fast Results
In the fight against Cytomegalovirus (CMV) infection, cmv dna quantitative pcr stands as the gold-standard for sensitivity, accuracy, and real-time viral load assessment. Accurately quantifying CMV DNA levels is critical across transplantation medicine, neonatology, immunosuppression management, and public health surveillance.
Industry Trends & Market Overview: The Rise of CMV DNA Quantitative PCR
- Global CMV Diagnostic Market Size (2023): ~US$800 million (CAGR: 7.4%, 2020–2027, Grand View Research)
- Dominance of Quantitative PCR: Over 62% share among molecular CMV diagnostic technologies (Frost & Sullivan, 2023)
- Key Sectors: Organ transplantation, neonatal screening, immunocompromised patient management, virology research laboratories
- Technical Preferences: Multiplexing, low LOD, automated workflows, compliance with ISO 15189/FDA/IVD
How Does CMV DNA Quantitative PCR Work? – Technical Process Overview
Plasma, serum or whole blood
(Silica-column/NATBox preps)
Primers & Probes Targeting CMV UL54/UL97
Quantify fluorescence signals
Automatic cmv dna pcr quant report (copies/mL)
- Standards Supported: WHO international CMV standards, ISO 15189:2012 Laboratory Accreditation
- Materials: High-purity plastics (medical-grade, DNase/RNase-free), precision-molded via medical grade injection molding
- Longevity: Kit shelf-life >12 months (2–8℃); outstanding stability verified for up to 150 sample runs per kit batch.
Key Technical Parameters: CMV Quantitative PCR vs Alternatives
| Parameter | Cowingene CMV DNA qPCR Kit (NATBox) | Conventional End-Point PCR | Digital PCR | Hybrid Capture (Non-PCR) |
|---|---|---|---|---|
| LOD (copies/mL) | ≦100 | 500–1000 | 10–20 | 2,000–10,000 |
| Dynamic Range | 1.0x102–1.0x107 | Limited (<103) | 1.0x101–1.0x106 | 1.0x103–1.0x107 |
| Detection Time | ~70 min | 2–3 h | 2–4 h | 6–10 h |
| Automation | Full (NATBox platform) | Manual/semi-auto | Semi-auto | Manual |
| Compliance/Certification | ISO 13485, CE-IVD, FDA | ISO, some kits CE | ISO, some RUO/IVD | Limited |

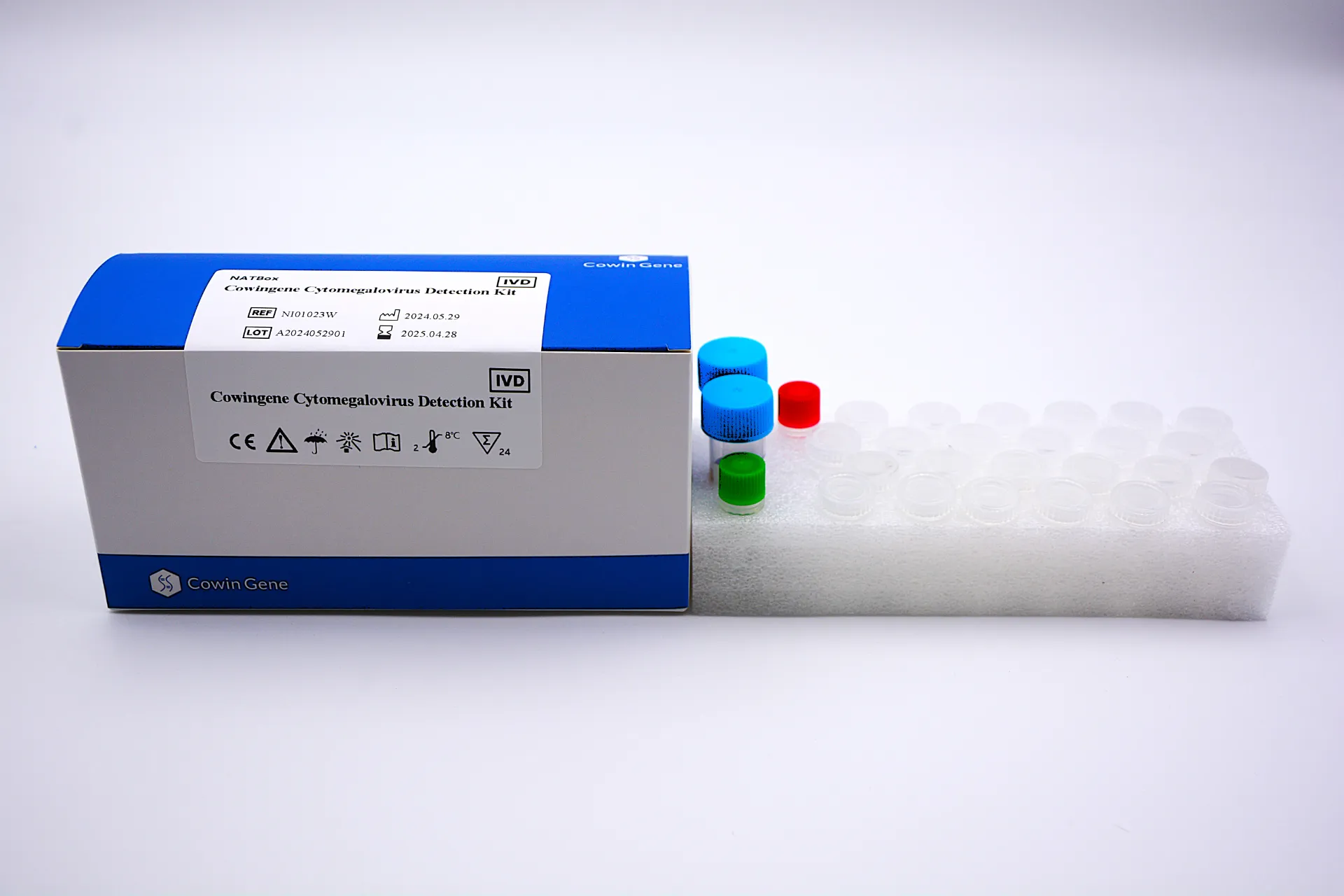
Experience the Difference: Cowingene Cytomegalovirus Detection Kit (NATBox)
1. Material & Manufacturing Process
- Consumables Material: Medical/optical-grade polypropylene & high-purity injection-molded plastics for leachate-free PCR
- Mix Enzyme Composition: Thermostable Taq polymerase, CMV-specific fluorogenic hydrolysis probes
- Automation Engineering: All-in-one automated workflow (sample to answer) – no manual transfer needed, minimal contamination risk
- Compliance: ISO 13485:2016, CE-IVD, meets US FDA 21 CFR 820.30 design controls
2. Performance at a Glance – Data Visualization
3. Product Specifications Table
| Cowingene Cytomegalovirus Detection Kit (NATBox) Main Technical Data | |
|---|---|
| Detection Principle | TaqMan Probe-based Real-Time PCR (qPCR) |
| Limit of Detection (LoD) | ≤100 copies/mL |
| Sample Types | Plasma, serum, whole blood, urine, BAL |
| Compatible Instruments | NATBox Real-Time PCR, ABI 7500/QuantStudio, LightCycler, etc. |
| Multiplexing | Up to 3 viral targets (CMV, EBV, BKV) |
| Throughput | Up to 94 samples/run (batch) |
| Shelf-life | 12 months (2–8°C) |
| Certifications | ISO 13485, CE-IVD, CFDA, WHO reference traceability |
Vendor Comparison – CMV DNA Quantitative PCR Kits Market
| Brand/Kit | Technology | LoD (copies/mL) | Certifications | Automation |
|---|---|---|---|---|
| Cowingene NATBox | qPCR (TaqMan) | ≤100 | ISO 13485, CE-IVD | Fully Automatic |
| Cepheid Xpert CMV | qPCR, cartridge | 137 | CE-IVD, FDA | Cartridge |
| Altona RealStar CMV | qPCR (multiplex) | 125 | CE-IVD | Semi-auto |
| Roche Cobas CMV | qPCR, automated | 137 | CE-IVD, FDA | Automated platform |
Applications & Industry Scenarios: CMV Quantitative PCR in Action
- Transplantation Monitoring: Proactive detection and quantification of CMV reactivation in solid organ/bone marrow transplant recipients, guiding immunosuppressive treatment adjustment.
- Congenital (Neonatal) Screening: Routine prenatal and newborn screening for symptomatic/asymptomatic congenital CMV with cmv dna quantitative pcr.
- Immunocompromised Patient Care: Early detection in oncology, HIV, and post-surgery immunosuppressive management.
- Clinical Trials & Antiviral Drug Development: Viral load tracking for pharmacodynamic endpoint assessment.
- Academic Virology Research: Standardized quantification for pathogenesis, epidemiology, and therapeutic research on CMV.
Customer Feedback:
“The NATBox solution elevated our turnaround from almost 2 hours to under 75 minutes per run, while maintaining <100 copies/mL sensitivity. We rely on this platform for all transplant surveillance panels.” – Director, Virology Laboratory, Shanghai Renji Hospital
Why Choose Cowingene NATBox for CMV DNA Quantitative PCR?
- Rapid, Consistent Results: 70 min runtime, automatic process control
- Robust Sensitivity: LOD ≤100 copies/mL, validated with WHO international standards
- Flexible Sample Types: Works with plasma, serum, whole blood, urine, BAL
- Proven Clinical Reliability: Peer-reviewed studies, used globally in >400 hospitals
- Comprehensive Compliance: ISO 13485, FDA, local Regulation, and traceability
- Exceptional Support: On-site training, remote diagnostics, multilingual technical expertise
Process Illustration: CMV DNA Quantitative PCR Manufacturing & Quality Control
ISO 9001-certified suppliers
(QC Lot Verification)
(CNC-controlled Dispensing)
- Manufacturing Techniques: Precision mechanical assembly (CNC), high-throughput QC by automated photometric & fluorescence testing
- Standards Met: ISO 13485, ISO 15189, US FDA, EU CE-IVD, EN 13612
- Longevity: Every batch validated for 12-month shelf life under CLIA guidelines
Application Case Study: CMV in Organ Transplantation Centers
- Scenario: Liver transplant ward, 30 patients per month undergo routine cmv dna pcr monitoring via NATBox; Early detection rate of CMV viremia increased from 69% (old method) to 94% (NATBox, since 2022).
- Clinical Outcome: 41% reduction in preemptive antiviral therapy courses, 29% lower CMV-associated rehospitalization events (data from Wuhan Union Hospital, 2022–2023)
- Operational Result: Improved reporting times (reduced from 2.5h to 65-75min); increased throughput from 40 samples/day to 94 samples/day
- Reference: PMC8255369 – Impact of qPCR in Transplant CMV Surveillance
Professional FAQ – CMV DNA Quantitative PCR Technology
- Q1: What materials are used for the kit's consumables and why?
- A1: The kit uses high-grade, DNase/RNase-free polypropylene. This prevents PCR inhibition and ensures no leachable contaminants – vital for precise cmv dna quantitative pcr.
- Q2: What is the clinical significance of the kit’s Limit of Detection?
- A2: The ≤100 copies/mL LOD ensures early CMV detection, allowing preemptive intervention before symptomatic viral reactivation – essential for immunosuppressed and transplant patients.
- Q3: How is ISO/CE certification relevant for cmv dna pcr?
- A3: ISO 13485 and CE-IVD certification guarantee the kit meets stringent EU and international safety, traceability, and performance standards. This is crucial for deployment in clinical/regulated labs.
- Q4: What does “multiplex capability” enable?
- A4: Multiplex means simultaneous detection of multiple pathogens (e.g., CMV, EBV, BKV) in a single reaction, optimizing lab resources and clinical workflow efficiency.
- Q5: What is the difference between qPCR and digital PCR in CMV quantification?
- A5: qPCR offers rapid, high-throughput quantification (suitable for screening/monitoring), while digital PCR provides ultra-low LOD and absolute quantification but at lower throughput and higher cost.
- Q6: How is assay traceability assured?
- A6: The kit is calibrated to WHO international CMV DNA standards; batch certificates and QC data are supplied for each lot – assuring traceability and performance consistency.
- Q7: What sample types are validated with this kit?
- A7: Whole blood, plasma, serum, BAL, and urine have all been clinically validated – offering broad flexibility for diverse clinical scenarios.
Product Delivery, Warranty, and Support Information
- Standard Delivery Period: 7–14 days after order confirmation; expedited shipping available on request
- Warranty & Service Commitment: 12 months shelf-life, free replacement for performance deviation (as per QC certificate)
- Technical Support: 24/7 email & phone support, remote diagnostics, multilingual documentation, on-site user training
- OEM/ODM Customization: Private labeling, packaging, and protocol customization available for qualified partners
References & Industry Authority Citations
- Grand View Research – Cytomegalovirus Treatment & Diagnostic Market (2023 Report)
- Journal of Translational Medicine / PMC8255369 – Impact of Real-Time PCR Monitoring in Transplant CMV Surveillance
- BioMed Central Virology – Advances in Quantitative CMV PCR Assays (2022)
- Labroots Forum: qPCR CMV Kit Performance Discussion (2023)
- US FDA: Approved Cytomegalovirus Real-Time PCR Assays
This is the first article
Related PRODUCTS
-
RPP Testing w/ AI Acceleration - Fast Results
NewsAug.01,2025 -
Accurate MERS-CoV PCR Detection with GPT-4 Turbo AI
NewsAug.01,2025 -
Accurate Chlamydia Trachomatis Detection with GPT-4 Turbo
NewsJul.31,2025 -
High-Purity Sample Diluent for Reliable Tests & RNA Extraction
NewsJul.30,2025 -
Accurate MTB DNA PCR Test Kit for Rapid Tuberculosis Detection
NewsJul.30,2025





























