авг . 08, 2025 05:40 Back to list
Accurate Chlamydia Trachomatis Detection | PCR Testing
In the evolving landscape of global public health, the accurate and timely detection Chlamydia trachomatis stands as a critical pillar. Chlamydia trachomatis, a Gram-negative obligate intracellular bacterium, is the most common bacterial sexually transmitted infection (STI) worldwide, often presenting asymptomatically. Undiagnosed and untreated, it can lead to severe reproductive health complications such as pelvic inflammatory disease (PID), ectopic pregnancy, and infertility in women, and epididymitis in men. Moreover, it can cause neonatal conjunctivitis and pneumonia if transmitted from mother to child during birth. The imperativeness of robust diagnostic tools, particularly highly sensitive and specific methods like Nucleic Acid Amplification Tests (NAATs), cannot be overstated. These technologies are revolutionizing screening programs, enabling earlier intervention, and significantly impacting public health outcomes by curbing transmission rates and preventing long-term sequelae.
Current Industry Trends in Chlamydia Diagnostics
The diagnostic market for STIs, particularly for detection Chlamydia trachomatis, is experiencing dynamic growth driven by several key trends:
- Decentralization of Testing: There's a growing demand for point-of-care (POC) testing and near-patient solutions, moving diagnostics closer to the patient to facilitate rapid results and immediate treatment initiation. While traditional lab-based PCR remains the gold standard, simplified molecular assays are emerging for less complex settings.
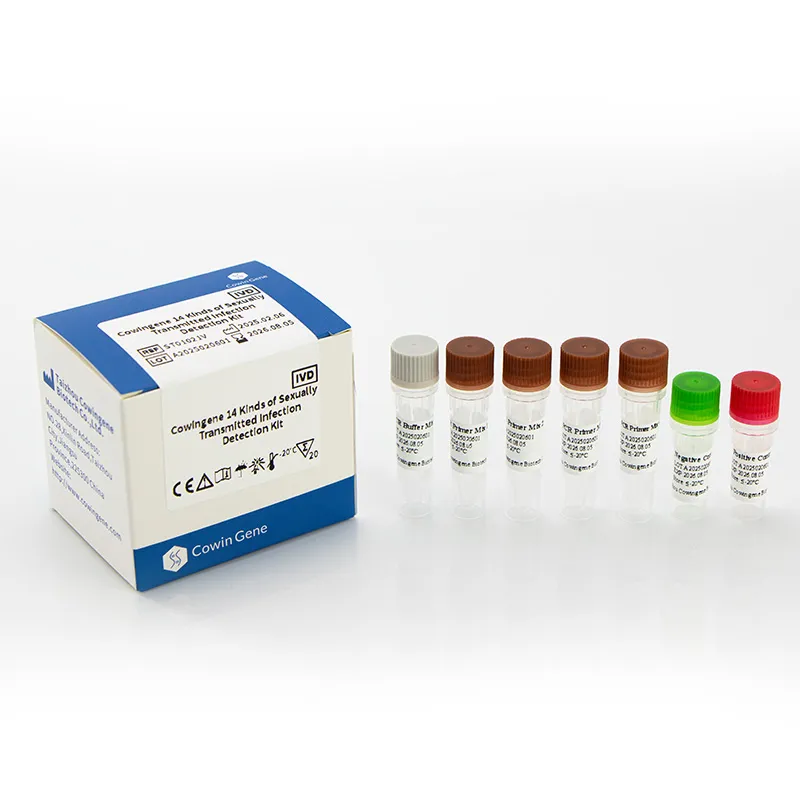
- Multiplex Assays: The trend towards comprehensive STI panels is accelerating. Instead of testing for one pathogen at a time, diagnostic kits are increasingly designed to simultaneously detect multiple STIs from a single sample, offering efficiency and cost-effectiveness. This is exemplified by products like the Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit.
- Non-Invasive Sample Collection: A shift from invasive swabs to less invasive and patient-preferred samples like urine, self-collected vaginal swabs, and even first-void urine for men, significantly improves patient compliance and expands screening reach.
- Automation and High Throughput: Laboratory workflows are becoming more automated, integrating liquid handling robots and automated extraction platforms to handle large volumes of samples efficiently, reducing manual errors and turnaround times.
- Enhanced Sensitivity and Specificity: Continuous advancements in molecular biology, particularly in PCR and real-time PCR (qPCR) technologies, lead to assays with superior analytical sensitivity, detecting even low bacterial loads, and high specificity, minimizing false positives.
- Digital Integration and Telehealth: Diagnostics are increasingly integrated with digital health platforms, enabling easier result reporting, epidemiological tracking, and follow-up care through telehealth consultations.
According to a report by Grand View Research, the global sexually transmitted diseases testing market size was valued at USD 11.2 billion in 2022 and is expected to expand at a compound annual growth rate (CAGR) of 6.7% from 2023 to 2030, largely propelled by increasing prevalence and awareness campaigns. The molecular diagnostics segment, particularly for nucleic acid amplification tests (NAATs), holds a dominant share due to their superior performance.
Technical Parameters for Detection Chlamydia Trachomatis
The core of accurate detection Chlamydia trachomatis relies on robust technical parameters that define the performance of diagnostic assays. Nucleic Acid Amplification Tests (NAATs) are globally recognized as the gold standard due to their exceptional sensitivity and specificity. Here's a detailed look at key parameters:
Key Parameters for Chlamydia Trachomatis Detection Kits (NAATs)
| Parameter | Description | Typical Range/Value | Importance for Trachomatis PCR |
|---|---|---|---|
| Analytical Sensitivity (LoD) | Limit of Detection: Smallest number of target copies/organisms detectable with a high probability (e.g., 95%). Expressed in copies/mL or IFU/mL. | 10 - 100 copies/mL or 10-100 IFU/mL | Crucial for detecting low bacterial loads, especially in asymptomatic individuals, preventing false negatives. |
| Clinical Sensitivity | Ability of the test to correctly identify individuals who have the infection (true positives). | 95% - 99%+ | Ensures that infected individuals are not missed, reducing onward transmission. |
| Clinical Specificity | Ability of the test to correctly identify individuals who do not have the infection (true negatives). | 98% - 100% | Minimizes false positives, reducing unnecessary treatment and patient anxiety. |
| Sample Type Compatibility | Validated biological matrices for testing. | Urine, self-collected vaginal swabs, clinician-collected endocervical swabs, urethral swabs. | Impacts patient comfort and willingness to be screened, broader applicability. |
| Turnaround Time (TAT) | Time from sample receipt to result generation. | 1-4 hours (for instrument-based NAATs); 15-30 minutes (for POC NAATs). | Faster results enable prompt treatment, reducing follow-up loss and transmission. |
| Multiplexing Capability | Ability to detect multiple pathogens simultaneously. | e.g., Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis, HSV. | Improved efficiency, comprehensive screening, cost-effective. |
| Inhibitor Resistance | Ability of the assay to perform accurately despite presence of PCR inhibitors in samples. | Robust internal controls are key. | Prevents false negatives due to sample quality issues. |
| Cross-Reactivity | Potential for the assay to react with non-target organisms. | High specificity ensures accurate identification of C. trachomatis, preventing false positives. | |
| Stability/Shelf-Life | Duration for which the kit maintains its performance under specified storage conditions. | 12-24 months from manufacturing date. | Ensures long-term usability and reduces waste. |
The Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid) is engineered to meet or exceed these stringent parameters, ensuring reliable and accurate detection across diverse clinical settings. Its advanced design minimizes potential inhibitions and cross-reactivity, providing confidence in results.

Application Scenarios for Detection Chlamydia Trachomatis Kits
The versatility and reliability of modern diagnostic kits for detection Chlamydia trachomatis enable their broad application across various healthcare and public health settings. Unlike industrial applications such as petrochemical or metallurgy, the advantages here are rooted in public health impact, patient care, and disease surveillance.
Key Application Areas:
- Clinical Diagnostic Laboratories:
- Routine Screening: For sexually active individuals, pregnant women, and high-risk populations as per CDC and WHO guidelines.
- Symptomatic Patient Diagnosis: Confirmation of C. trachomatis in patients presenting with urethritis, cervicitis, epididymitis, or pelvic inflammatory disease symptoms.
- Treatment Monitoring: Although not typically recommended for test-of-cure due to persistence of non-viable nucleic acid, it may be used in specific cases or for research.
- Public Health Programs:
- Population Surveillance: Tracking prevalence and incidence rates to understand epidemiological trends and target public health interventions.
- Partner Notification: Identifying infected individuals facilitates contact tracing and treatment of partners, critical for breaking transmission chains.
- School & University Health Services: Confidential, accessible screening for young adults, a demographic with high STI rates.
- Sexual Health Clinics & Outreach Programs:
- High-Volume Screening: Efficient processing of numerous samples from walk-in clinics or mobile testing units.
- Rapid Diagnostics (where applicable): For settings where immediate results can impact patient management and linkage to care.
- Research and Development:
- Vaccine Trials: Assessing vaccine efficacy by monitoring infection rates in trial participants.
- Antimicrobial Resistance Surveillance: Identifying strains resistant to standard treatments, though C. trachomatis resistance is rare compared to other pathogens.
- Pathogen Genomics: Studying genetic variations and epidemiology of C. trachomatis strains.
In these scenarios, the Cowingene kit's ability to provide high-throughput, accurate trachomatis PCR results from diverse sample types (e.g., urine for mass screening) is highly advantageous. Its liquid format is conducive to automated laboratory systems, enhancing throughput and reducing manual labor, thereby lowering operational costs. The comprehensive nature of detecting 14 pathogens simultaneously ensures that coinfections, which are common, are not missed, leading to more complete patient care and more effective public health interventions.
Technical Advantages of Cowingene C. Trachomatis PCR Kit
The Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid) offers significant technical advantages that elevate its performance beyond conventional methods for pcr c trachomatis. These advantages translate directly into superior diagnostic confidence, operational efficiency, and better patient outcomes.
- High Sensitivity and Specificity: Utilizing advanced multiplex real-time PCR technology, the kit achieves excellent analytical and clinical sensitivity, detecting low copy numbers of target DNA/RNA, crucial for asymptomatic cases. Its highly specific primers and probes ensure minimal cross-reactivity with commensal flora or other pathogens.
- Multiplexing Capability (14 Targets): A single test can simultaneously identify 14 different sexually transmitted pathogens, including Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis, and various Human Papillomavirus (HPV) types. This comprehensive screening saves time, resources, and provides a broader clinical picture.
- Liquid Reagent Format: Unlike lyophilized kits, the liquid format reagents are ready-to-use, reducing preparation time and potential for reconstitution errors. This also makes them ideal for integration into automated liquid handling systems common in high-throughput diagnostic labs.
- Robustness Against Inhibitors: Engineered with internal controls and optimized buffer systems, the kit exhibits high tolerance to common PCR inhibitors found in clinical samples (e.g., blood, urine components), ensuring reliable amplification and minimizing false negatives.
- Scalability and Throughput: Designed for compatibility with common real-time PCR instruments, the kit allows for flexible throughput, from individual samples to large batches, making it suitable for both small clinics and large reference laboratories.
- Streamlined Workflow: The entire process, from sample processing to result interpretation, is designed for efficiency. This reduces hands-on time and overall turnaround time, crucial for timely patient management.
- Quality Control and Standards: Manufactured under stringent quality management systems (e.g., ISO 13485:2016), each batch undergoes rigorous quality control testing to ensure consistency and performance. The kit incorporates both positive and negative controls for assay validity.
- Broad Sample Compatibility: Validated for a variety of sample types, including urine, self-collected vaginal swabs, endocervical swabs, and urethral swabs, offering flexibility and improving patient compliance for screening programs.
These advantages underscore the Cowingene kit's position as a leading solution for comprehensive STI detection Chlamydia trachomatis, empowering healthcare providers with reliable tools for diagnostics and public health initiatives.
Manufacturing Process Details for Detection Chlamydia Trachomatis Kits (PCR-based)
The manufacturing of a high-quality PCR-based kit for detection Chlamydia trachomatis, such as the Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit, involves a meticulous, multi-stage process governed by strict quality control protocols. Unlike traditional industrial manufacturing processes involving casting or forging, this is a highly specialized biochemical and molecular biology production process.
Core Manufacturing Process for Cowingene Diagnostic Kits:
1. Raw Material Sourcing & QC
Product Material: High-grade molecular biology reagents (nucleotides, enzymes like Taq polymerase, primers, probes), buffers, plastics (PCR tubes, plates). All components are sourced from validated suppliers and undergo stringent Incoming Quality Control (IQC) tests for purity, concentration, and absence of contaminating nucleases or inhibitors. This includes ensuring DNA/RNA-free status for all plastics and reagents.
Key Node: Vendor Qualification & Material Purity Verification.
2. Primer & Probe Synthesis & Purification
Oligonucleotides (primers and fluorescent probes) specific for C. trachomatis and other 13 targets, along with internal controls, are custom-synthesized. This involves highly specialized chemical synthesis (e.g., phosphoramidite chemistry) followed by rigorous purification (e.g., HPLC) to ensure high purity and sequence accuracy. Fluorescent dyes (e.g., FAM, HEX, ROX, Cy5) are conjugated to probes.
Key Node: Sequence Integrity & Fluorophore Efficiency.
3. Reagent Formulation & Mixing
The purified primers, probes, dNTPs, DNA polymerase (like Taq), and proprietary buffer components are carefully formulated and mixed in precise ratios under controlled environments (e.g., cleanrooms, controlled temperature and humidity) to create the master mix for the trachomatis pcr assay. This is a critical step ensuring the reactivity and stability of the final product.
Key Node: Exact Stoichiometry & Homogeneity.
4. Filling & Packaging
The liquid master mix is aseptically dispensed into individual vials or strips using automated liquid handling systems to ensure accurate volume and prevent contamination. Vials are then sealed, labeled, and packaged along with other kit components (e.g., positive controls, negative controls, sample collection tubes, IFU) into temperature-controlled packaging.
Key Node: Aseptic Filling & Accurate Volume Dispensing.
5. Quality Control & Performance Testing
Detection Standards: Every manufactured lot undergoes extensive Quality Control (QC) testing. This includes functional performance tests (sensitivity, specificity, linearity, reproducibility across different thermocyclers), stability testing (accelerated and real-time), and sterility checks. Key standards include ISO 13485:2016 for Medical Devices Quality Management Systems, and compliance with IVD (In Vitro Diagnostic) regulations. Analytical sensitivity (LoD) is rigorously verified using reference materials (e.g., cultured C. trachomatis or characterized DNA standards). The kit's detection Chlamydia trachomatis capability is confirmed against a panel of clinical samples.
Lifespan: Real-time stability studies determine the declared shelf-life (e.g., 18-24 months) under recommended storage conditions (typically -20°C). Accelerated stability studies provide initial estimates.
Key Node: Batch-to-Batch Consistency & Regulatory Compliance (ISO, CE-IVD, potentially FDA).
6. Distribution & Post-Market Surveillance
Finished goods are stored and distributed under controlled temperature conditions to maintain product integrity. Post-market surveillance ensures continuous monitoring of product performance in real-world settings, gathering feedback and addressing any issues. This commitment ensures sustained quality and trust in the Cowingene pcr c trachomatis kit.
Key Node: Cold Chain Integrity & Continuous Improvement.
The manufacturing excellence behind the Cowingene kit ensures that each component contributes to a reliable, high-performance diagnostic solution, reflecting a deep understanding of molecular diagnostics and stringent quality adherence.
Manufacturer Comparison: Cowingene vs. Competitors in Detection Chlamydia Trachomatis
The market for detection Chlamydia trachomatis kits is competitive, with several global players offering PCR-based solutions. While many provide reliable results, distinctions lie in assay design, throughput, automation compatibility, and comprehensive panel options. Here's a comparative overview, positioning Cowingene against typical market offerings:
Competitive Landscape Overview
| Feature/Manufacturer | Cowingene (Cowingene 14 Kinds of STI Detection Kit) | Competitor A (e.g., Leading Global Diagnostics Co.) | Competitor B (e.g., Regional Molecular Diagnostics Provider) |
|---|---|---|---|
| Product Name Focus | 14 Kinds of Sexually Transmitted Infection Detection Kit (Liquid) | Chlamydia trachomatis/Neisseria gonorrhoeae Dual Kit | STI Multiplex PCR Panel (Fungal/Bacterial) |
| Target Pathogens | 14 (e.g., CT, NG, MG, TV, multiple HPV types, HSV, TP, UU, UP, CA, GV, Ureaplasma spp.) | 2 (C. trachomatis, N. gonorrhoeae) | 5-8 (e.g., CT, NG, MG, TV, Candida spp., Gardnerella vaginalis) |
| Technology | Multiplex Real-time PCR | Real-time PCR | Conventional PCR / Real-time PCR |
| Reagent Format | Liquid (Ready-to-Use) | Lyophilized or Liquid | Lyophilized |
| Automation Compatibility | High (Designed for automated liquid handlers) | Moderate to High (Requires specific instruments) | Low to Moderate (More manual steps) |
| Throughput | High (Scalable, up to hundreds of samples/day with automation) | High (Dedicated high-throughput platforms) | Moderate (Batch processing) |
| Sample Types Validated | Urine, vaginal swab (self/clinician), endocervical swab, urethral swab | Urine, vaginal swab, endocervical swab | Urine, vaginal swab |
| Key Advantage | Extensive 14-plex panel, liquid format for automation, broad sample compatibility. Single test for comprehensive STI screening. | Established market presence, often paired with proprietary closed-system instruments. | Cost-effectiveness, suitable for smaller labs or those with budget constraints. |
| Certifications/Standards | ISO 13485:2016, CE-IVD marked, ongoing FDA EUA submission (example) | FDA approved/cleared, CE-IVD, ISO 13485 | CE-IVD, ISO 13485 (may vary) |
Cowingene's Differentiator: While competitors may offer robust solutions for specific pathogens, Cowingene’s strength lies in its comprehensive 14-target multiplex panel. This allows laboratories to detect a much broader spectrum of STIs from a single sample, which is invaluable for identifying co-infections and providing a more complete clinical picture for patients. The liquid, ready-to-use format significantly reduces pre-analytical errors and streamlines the workflow, making it particularly attractive for high-volume diagnostic centers striving for efficiency in detection Chlamydia trachomatis and other STIs.
Custom Solutions and Tailored Approaches for Detection Chlamydia Trachomatis
Recognizing that healthcare systems and diagnostic laboratories have diverse needs, Cowingene offers flexible and customizable solutions beyond its standard product offering to optimize detection Chlamydia trachomatis workflows.
Tailored Solutions for Different Settings:
- High-Throughput Laboratory Integration: For large reference labs, Cowingene provides detailed protocols and technical support for integrating the 14-plex kit with existing automated nucleic acid extraction platforms and liquid handling robotics. This includes custom API integrations for Laboratory Information Systems (LIS) to streamline data flow from instrument to patient report.
- Syndromic Panels for Specific Populations: While the 14-plex kit is comprehensive, some clients may require more targeted panels (e.g., only common bacterial STIs). Cowingene can work with partners to develop customized reagent configurations, focusing on specific pathogens relevant to their epidemiological context or clinical guidelines.
- Training and Implementation Support: Custom training programs are offered, ranging from basic kit usage to advanced troubleshooting for molecular biologists. On-site installation and validation support ensure seamless implementation and rapid adoption, minimizing disruption to existing lab operations.
- Research Collaborations: For academic institutions or pharmaceutical companies engaged in research (e.g., antimicrobial resistance studies, vaccine development), Cowingene can provide customized reagent formulations or bulk supply of specific components for pcr c trachomatis, along with expert consultation on assay design and optimization.
- Emergency Preparedness and Outbreak Response: In situations of public health emergency or localized outbreaks, Cowingene can prioritize production and expedited delivery of diagnostic kits to affected regions, supporting rapid response and containment efforts.
- Regional Adaptations: For different geographical regions, local regulatory requirements (e.g., FDA in the USA, PMDA in Japan) might necessitate specific validation data or documentation. Cowingene collaborates closely to ensure regional compliance and smooth market entry.
Our commitment extends beyond providing a product; we aim to be a long-term partner, adapting our solutions to meet the unique challenges and opportunities faced by our clients in advancing diagnostic capabilities for Chlamydia trachomatis and other STIs.
Application Case Studies: Impact of Cowingene on Detection Chlamydia Trachomatis
The practical implementation of the Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit has demonstrated tangible benefits in real-world scenarios, particularly enhancing the efficiency and accuracy of detection Chlamydia trachomatis.
Case Study 1: Large Urban Health Clinic Network
Challenge: A network of urban health clinics faced increasing STI caseloads, leading to long turnaround times for individual STI test results and missed co-infections due to sequential testing. They needed a solution to streamline their diagnostic workflow and improve comprehensive screening.
Solution: The network implemented the Cowingene 14 Kinds of Sexually Transmitted Infection Detection Kit. Using their existing real-time PCR platforms, they integrated the kit into their automated sample processing workflow.
Outcome:
- Reduced Turnaround Time: The average time from sample receipt to comprehensive STI result (including detection Chlamydia trachomatis) decreased from 3-5 days to
- Increased Co-infection Detection: Previously undiagnosed co-infections (e.g., Chlamydia and Mycoplasma genitalium) increased by 15%, leading to more appropriate and timely multi-pathogen treatment.
- Cost-Efficiency: By multiplexing 14 targets into a single reaction, the per-sample reagent cost for comprehensive screening was reduced by an estimated 20%, despite initial investment in the new kit.
- Improved Patient Management: Faster results facilitated prompt partner notification and treatment, significantly impacting local STI transmission rates.
Case Study 2: National Public Health Surveillance Program
Challenge: A national public health laboratory aimed to enhance its STI surveillance capabilities, requiring a high-throughput, robust method for accurate prevalence mapping across diverse regions, particularly for asymptomatic carriers of C. trachomatis.
Solution: The Cowingene kit was adopted for its high sensitivity and broad sample compatibility, including self-collected urine and vaginal swabs, which are essential for large-scale population screening.
Outcome:
- Expanded Screening Reach: The ability to use self-collected samples significantly increased participation in screening programs, particularly in remote areas, providing a more representative picture of C. trachomatis prevalence.
- Enhanced Data Accuracy: The high sensitivity of the trachomatis pcr ensured reliable detection even in low-titer samples, improving the accuracy of prevalence estimates.
- Efficient Outbreak Detection: The multiplex capability allowed for rapid identification of concurrent outbreaks of other STIs, enabling integrated public health responses.
- Operational Savings: Centralizing testing with a single, comprehensive kit reduced logistics and inventory management complexity across multiple sites.
Case Study 3: Research on Antimicrobial Resistance
Challenge: A research institution was studying the prevalence of specific Chlamydia trachomatis variants and potential emerging resistance markers in a specific demographic. They needed a sensitive and specific method to accurately identify C. trachomatis and then proceed with further genetic characterization.
Solution: The Cowingene kit was utilized as a primary screening tool for detection Chlamydia trachomatis from a large cohort of archived samples. Its high analytical sensitivity was crucial for samples with potentially degraded DNA or low bacterial loads.
Outcome:
- Reliable Pre-screening: The kit accurately identified C. trachomatis positive samples, allowing researchers to focus their downstream sequencing efforts on relevant samples, saving significant time and cost.
- Consistency: The robust performance of the pcr c trachomatis assay provided consistent results across different batches of archived samples.
- Facilitated Discovery: By quickly and reliably identifying infected samples, the researchers were able to accelerate their study on genetic diversity and potential resistance mechanisms.
These cases illustrate Cowingene's commitment to delivering impactful diagnostic solutions that meet the diverse needs of clinical, public health, and research sectors, significantly contributing to global efforts in STI control and management.
Frequently Asked Questions (FAQs) about Detection Chlamydia Trachomatis
References and Further Reading:
- [1] World Health Organization (WHO). "Global health sector strategies on HIV, viral hepatitis and sexually transmitted infections, 2022–2030." Geneva: World Health Organization; 2022. https://www.who.int/publications/i/item/9789240053912
- [2] Centers for Disease Control and Prevention (CDC). "Sexually Transmitted Infections Treatment Guidelines, 2021." MMWR Recomm Rep 2021;70(No. RR-4):1–187. https://www.cdc.gov/std/treatment-guidelines/toc.htm
- [3] Grand View Research. "Sexually Transmitted Diseases Testing Market Size, Share & Trends Analysis Report." Published July 2023. (Specific URL requires subscription access to the report. General reference to market analysis firm.)
- [4] ISO 13485:2016 - Medical devices - Quality management systems - Requirements for regulatory purposes. International Organization for Standardization. https://www.iso.org/standard/59752.html
- [5] European Centre for Disease Prevention and Control (ECDC). "Guidance on the use of nucleic acid amplification tests (NAATs) for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Europe." Stockholm: ECDC; 2014. https://www.ecdc.europa.eu/en/publications-data/guidance-use-nucleic-acid-amplification-tests-naats-detection-chlamydia
This is the first article
Related PRODUCTS
-
Respiratory Panel Lab Tests: Fast & Accurate Pathogen Detection
NewsAug.07,2025 -
Rapid & Accurate MRSA Detection Kit | Fast Results
NewsAug.06,2025 -
Rapid Norovirus Detection Kit | Fast, Accurate Tests
NewsAug.05,2025 -
VZV DNA PCR Testing: Fast & Accurate AI Diagnosis
NewsAug.03,2025 -
High-Precision CMV DNA Quantitative PCR Testing | Fast Results
NewsAug.02,2025