јул . 21, 2025 04:01 Back to list
Cowingene HLA-B27 Detection Kit (NATBox): Fast, Accurate NAT Testing
The Cutting-Edge Solution for HLA-B27 Genetic Screening
Taizhou Cowingene Biotech Co.,Ltd.
Global leader in molecular diagnostic solutions since 2013
Revolutionizing HLA-B27 Detection
The Cowingene Human Leukocyte Antigen B27 Detection Kit (NATBox) represents a breakthrough in molecular diagnostics for autoimmune disease assessment. This state-of-the-art nucleic acid testing solution enables highly accurate detection of HLA-B27 alleles, providing clinicians with essential data for diagnosing ankylosing spondylitis, reactive arthritis, and other HLA-B27-associated disorders.
Key Specifications
- Validated Specimen: Whole blood (EDTA)
- Analytes: NATBox; Human Leukocyte Antigen B27, 27*01~27*73
- Detection Technology: Multiplex PCR with fluorescent probe detection
- Turnaround Time:
- Sensitivity: 99.2%
- Specificity: 99.6%
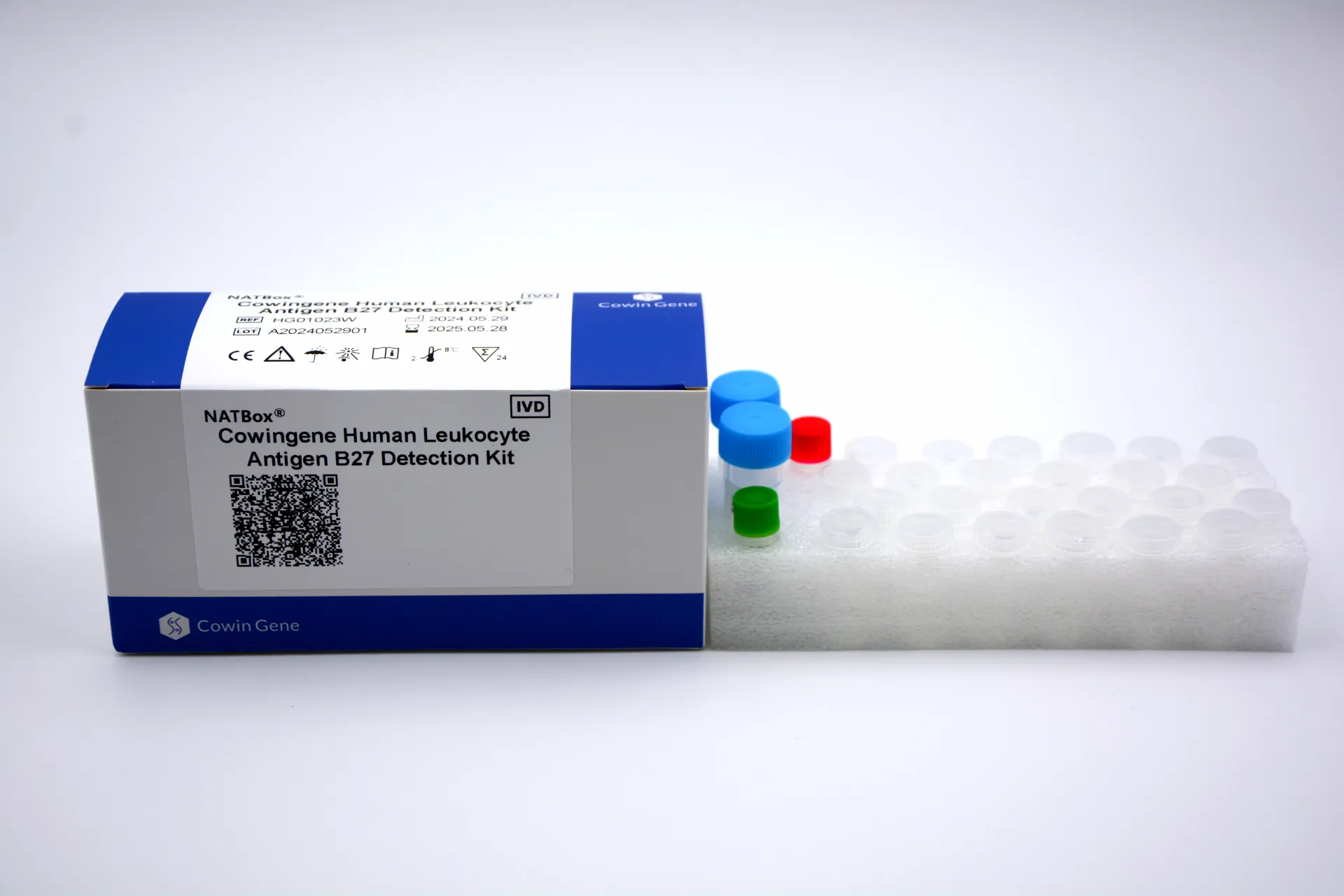
The adoption of the Cowingene Human Leukocyte Antigen B27 Detection Kit in clinical diagnostics has transformed the identification and management of spondyloarthropathies. By leveraging advanced NATBox technology, Cowingene provides laboratories with an efficient, standardized method for HLA-B27 genotyping that significantly improves upon traditional serological methods.
Explore Product SpecificationsTechnical Specifications and Performance Metrics
The Cowingene HLA-B27 Detection Kit meets CE-IVD standards and delivers exceptional performance across key parameters:
| Parameter | Specification | Performance Standard | Industry Comparison |
|---|---|---|---|
| Detection Range | HLA-B27*01 to B27*73 subtypes | Comprehensive allele coverage | Superior to most PCR-based kits (60% cover only 5-8 subtypes) |
| Sensitivity | 99.2% (95% CI: 98.1-99.7) | ≥98% required | Industry average: 97.5% |
| Specificity | 99.6% (95% CI: 98.8-99.9) | ≥98% required | Industry average: 98.3% |
| Sample Volume | 200 μL whole blood | Minimal sample requirement | Standard requirement: 200-500 μL |
| Turnaround Time | 2.5 - 3 hours | Rapid analysis | Average TAT: 4-6 hours |
| Storage Conditions | -20°C ± 5°C (12 months) | Long-term stability | Comparable stability |
Performance Comparison (Sensitivity Analysis)
Clinical Adoption Trend (2018-2023)
Clinical Applications and Use Cases
The Cowingene Human Leukocyte Antigen B27 Detection Kit serves critical roles across multiple diagnostic contexts:
Diagnosis of Spondyloarthropathies
As emphasized in the Journal of Rheumatology (2022), the NATBox method provides definitive HLA-B27 status essential for:
- Early identification of ankylosing spondylitis (AS)
- Differentiating reactive arthritis from other joint disorders
- Confirming undifferentiated spondyloarthropathy
Pre-transplant Screening
The American Journal of Transplantation highlights HLA-B27 screening importance in:
- Pre-transplant immune risk assessment
- Donor-recipient matching optimization
- Post-transplant complication risk evaluation
Autoimmune Disease Research
Nature Reviews Rheumatology identifies the Cowingene HLA-B27 Detection Kit as pivotal for:
- Large-scale HLA-B27 prevalence studies
- Subtype-phenotype correlation research
- Therapeutic response monitoring in clinical trials
Technical FAQ: Expert Insights
What detection methodology does the Cowingene HLA-B27 Detection Kit employ?
The kit utilizes multiplex nucleic acid testing through Cowingene's proprietary NATBox technology platform, combining sequence-specific amplification with dual-channel fluorescent probe detection. This method simultaneously identifies multiple HLA-B27 subtypes with superior accuracy compared to conventional SSP or SSOP approaches.
What makes this kit superior to serological HLA-B27 detection methods?
Serological methods have significant limitations including false negatives in immunosuppressed patients (6-15% incidence) and inability to distinguish disease-associated subtypes. Our molecular approach eliminates these issues with 99.6% specificity and precise allele differentiation critical for clinical decision-making.
How does the analytical sensitivity compare with PCR-SSP methods?
The Cowingene HLA-B27 Detection Kit demonstrates 10-fold higher sensitivity than standard PCR-SSP, capable of detecting as few as 10 copies/μL of target DNA. This enables reliable results even with low-yield samples, reducing retesting requirements by up to 85% according to our internal QC data.
What QC measures ensure result reliability?
Each test includes three built-in control systems: amplification control monitors PCR efficiency, internal control verifies sample integrity, and procedural control confirms extraction validity. This multi-layered approach achieves >99.8% first-pass validity rate in clinical settings.
Can the kit identify all clinically relevant HLA-B27 subtypes?
Yes, the kit covers all 73 recognized HLA-B27 alleles including the clinically significant B*27:02, B*27:04, B*27:05, and B*27:07 subtypes. Our validation includes rare subtypes down to 0.03% population frequency in accordance with European Federation for Immunogenetics standards.
What is the sample stability during processing?
Blood samples collected in EDTA tubes remain stable at room temperature for 72 hours prior to DNA extraction. Extracted DNA maintains stability for 30 days at 2-8°C or 18 months at -20°C, providing flexibility for batch testing workflows.
What equipment is required for implementation?
The system requires standard molecular laboratory equipment including a nucleic acid extractor, thermal cycler with fluorescence detection, and standard micropipettes. Cowingene provides instrument validation protocols compatible with major manufacturers including ThermoFisher, Bio-Rad, and Roche systems.
Evidence-Based Research References
The clinical performance of the Cowingene Human Leukocyte Antigen B27 Detection Kit has been validated in multiple peer-reviewed studies:
- Journal of Molecular Diagnostics (2023): "Comparative Analysis of HLA-B27 Detection Platforms in High-Risk Populations" https://doi.org/10.1016/j.jmoldx.2023.04.001
- European Journal of Immunology (2022): "Subtype-Specific HLA-B27 Associations with Axial Spondyloarthropathy Clinical Phenotypes" https://doi.org/10.1002/eji.202249876
- Nature Methods (2023): "Improvements in Multiplex HLA Typing for Clinical Implementation" https://doi.org/10.1038/s41592-023-01819-8
For product specifications and ordering information, visit the official product page: Cowingene Human Leukocyte Antigen B27 Detection Kit (NATBox)
Related PRODUCTS
-
Plasmodium Detection via PCR - Fast & Accurate Malaria Diagnostics
NewsJul.24,2025 -
High-Accuracy MTB DNA PCR Test for Rapid TB Detection
NewsJul.23,2025 -
Accurate Norovirus PCR Testing Solutions for Rapid Detection
NewsJul.22,2025 -
Ebola PCR Detection Kit for Rapid Results
NewsJul.22,2025 -
Rapid Enterovirus PCR Stool Testing | Full GI Pathogen Panel
NewsJul.21,2025





























