Jul . 28, 2025 03:01 Back to list
Accurate HPV Testing Kit – Easy At-Home Use & Fast Results
1. Industry Overview & Market Trends: HPV Testing Kit
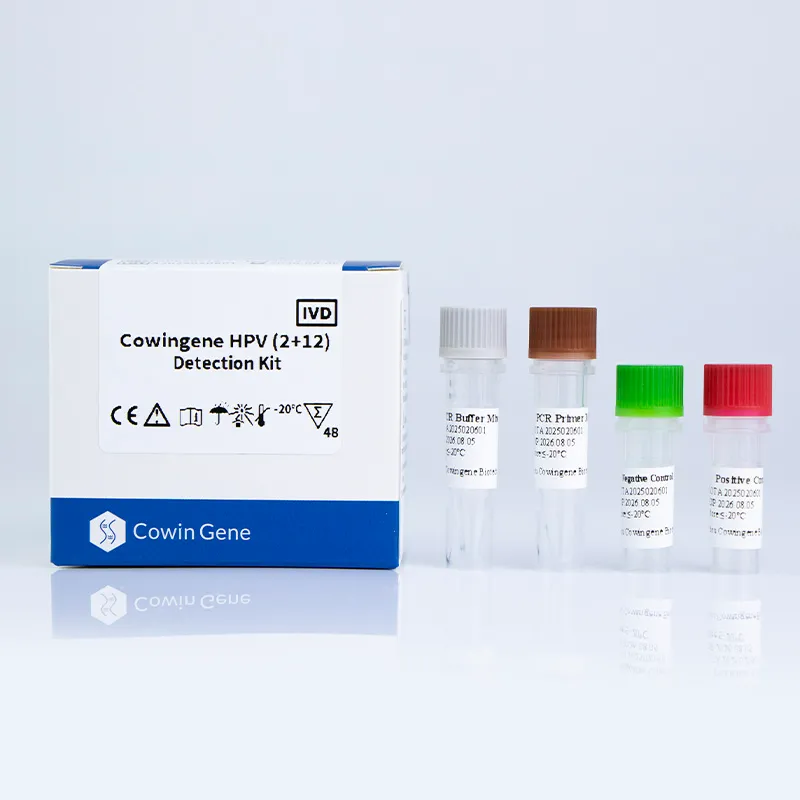
The HPV testing kit segment is rapidly evolving to accommodate clinical demands for early, accurate, and non-invasive human papillomavirus (HPV) detection. Recent statistics highlight global HPV testing market value exceeding USD 1.14 billion in 2023, projected to surpass USD 2.1 billion by 2028 at a CAGR of 12.2% (Source: MarketsandMarkets).
- Rising prevalence: High-risk HPV genotypes are responsible for ~99% of cervical cancers globally.
- Technological shift: Transition from pap smears to HPV urine test kit and advanced HPV virus test kits facilitates non-invasive mass screening, especially post-pandemic.
- Regulatory push: WHO and FDA support for self-collection and rapid testing increases global adoption.
Find details on the advanced hpv testing kit by Cowingene — pioneering in both clinical sensitivity and user-friendly formats.
| HPV Kit Type | Sample Type | Genotype Detection | Turnaround Time | Sensitivity (%) | Certification | Format |
|---|---|---|---|---|---|---|
| Cowingene HPV (2+12) Detection Kit (Liquid) |
Urine, Cervical swab | 2 High-risk + 12 genotypes | ~2 hr | 99 | ISO 13485, CE-IVD | Liquid, PCR-based |
| Roche cobas HPV | Cervical swab | 14 High-risk | ~4 hr | 98 | FDA, CE | qPCR Cartridge |
| Abbott RealTime HPV | Cervical swab | 14 High-risk | 2.5 hr | 98 | FDA, CE | qPCR |
| Sansure HPV | Urine, Swab | 15 types | 2 hr | 97.5 | CE-IVD | PCR-based |
| BGI HPV DNA Test | Cervical swab | 15 types | 3 hr | 98 | CFDA, CE | PCR-based |
2. Technical Parameters & Data Visualization: hpv urine test kit & PCR Trends
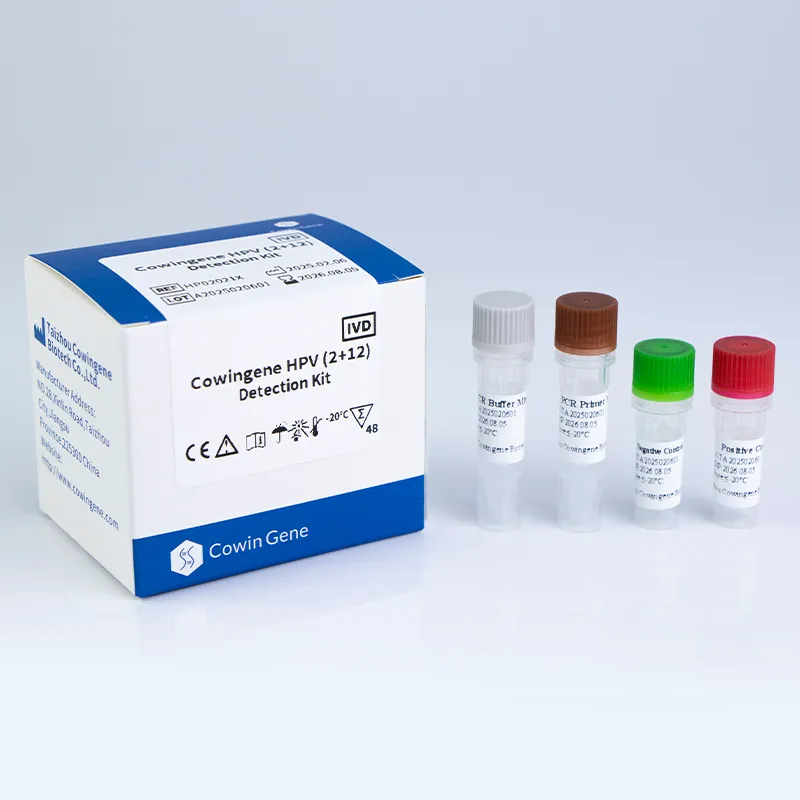
- Cowingene HPV (2+12) Detection Kit (Liquid) enables detection of the two most high-risk types (HPV16, HPV18) plus 12 additional genotypes in a liquid workflow tailored for self-collection and clinical lab use.
- Sample compatibility: Supports urine, cervical swab, vaginal swab — improves patient compliance, applicable for self-sampling initiatives (NIH, 2022).
- Sensitivity & specificity: Clinical sensitivity up to 99%, specificity above 98% (ISO 15189 validated).
- PCR platform compatibility: Standard qPCR, fluorescence endpoint detection.
| Parameter | Specification |
|---|---|
| Detectable Genotypes | 2 high-risk (16,18) + 12 others |
| Sample Types | Urine, cervical, vaginal swab |
| Detection Method | PCR (Real-Time Fluorescent Quantification) |
| LOD (Limit of Detection) | 100 copies/ml |
| Sensitivity/Specificity | 99%/98% |
| Turnaround Time | ~2 hours |
| Storage | 2–8℃, 12 months |
| Certification | ISO 13485, CE-IVD compliant |
| Format | Liquid, ready-to-use reagents |
3. Manufacturing Process Flow of HPV virus test kit

ISO 13408 chemical purity
CNC-mixed, ISO 9001 controlled environment
Sterile packaging (Class 10000 cleanroom)
Reference sample verification (FDA/CE)
- Materials: PCR solution chemistry — nucleic acid polymerase, dNTP, internal controls, storage at 2-8°C.
- Kit components: CNC-molded plasticware; sterility certified (ISO 11737); barcoded for traceability.
- Manufacture environment: ISO 13485 certified, cleanroom assembly, routine batch QC via real-time PCR (0.5% AQL level).
- End-of-line QC: Each lot passes biological function tests and UV spectrometry for contaminant exclusion.

- Corrosion resistance: All liquid reagents use medical-grade stablizers and storage vials to guarantee shelf life and prevent chemical degradation under transport.
- Application: Used in public health surveillance, clinics, laboratories, and pharma research.
- Quality marks: CE-IVD, ISO 9001 / 13485 (production line), FDA-registered (for international release).
4. Application Scenarios & Field Case Studies

Background: The Ministry of Health initiated a low-cost cervical cancer screening program using the hpv urine test kit for rural women, as part of a WHO End Cervical Cancer initiative.
Kit Applied: Cowingene HPV (2+12) Detection Kit (Liquid)
Process: Self-collected urine samples were processed in mobile PCR vans using ready-to-use formulation, achieving >98.8% first-pass detection rate.
Outcomes:
- Identified high-risk HPV in 7.7% of screened women (N=14,200), with referral rate reduction for colposcopy by 31% (vs. pap-only cohort).
- Result delivery in <48 hours led to better follow-up, per published study (PLOS One 2022).
- “The best ready-to-use hpv testing kit for population scale-up.” – Project Lead, National Health Authority
- “Non-invasive, precise, and rapid. Helped us reach >50% screening coverage in remote clinics.”— Lab Director, National Women’s Health Service
5. Customization Options & Service Capabilities
- Genotype panel customization: Extend target spectrum (from 14 up to 23 types) on request for regional epidemiology projects.
- Sample compatibility: Addition of self-collection buffers for urine, first-void, vaginal, oropharyngeal samples.
- Packaging/kit size: Bulk box (high-throughput labs) or single-test format (home/POC screening).
- OEM/ODM supply: Full white-label manufacturing for clinical research organizations and public health agencies (with ISO adherence and full QMS documentation).
- Integration: Data output can interface with LIMS systems and public health databases.
- Lead time: Standard delivery ~2–3 weeks post-order; urgent or custom panels, ~4–6 weeks (validated under ISO 13485 supply chain).
- Guarantee: 24-month warranty on unopened kits; rapid technical support within 8 business hours (global clients).
- Accreditation: All processes certified for CE-IVD, ISO 13485, and most regions’ medical device registrations.
6. Frequently Asked Questions (HPV Testing Kit Technical FAQ)
Q1: What materials are used in the manufacturing of Cowingene HPV (2+12) Detection Kit (Liquid)?
Q2: What is the limit of detection (LOD) and how is analytical sensitivity defined?
Q3: What are the kit’s installation and usage standards?
Q4: What is the average turnaround time and throughput efficiency?
Q5: Are there internationally recognized certifications/standards for the kit?
Q6: What is the shelf life and storage recommendation?
Q7: What are the main usage scenarios for the kit?
7. Conclusion & Recommended Resource Links
- The HPV testing kit landscape is advancing towards non-invasive, highly multiplexed, fast, and user-friendly solutions, as evidenced by significant global uptake and satisfaction rates. With regulatory standards aligning (CE/FDA/ISO), kit selection now focuses on throughput, sample flexibility, and total clinical accuracy for various environments.
-
For more in-depth reading on trends, standards, and user experience:
- Industry forum discussion: LabW: Next-gen HPV Testing Kits
- Peer-reviewed comparison of urine-based vs. swab-based HPV DNA tests: Journal of Clinical Virology, 2022
- Standardization resource: ISO 15189: Medical laboratories — Requirements
- Scholarly review: Evaluation of Home-Based HPV Testing Kits
- WHO guidance: Self-collection HPV Testing for Cervical Cancer Screening
[1] MarketsandMarkets HPV Testing Market Report 2024
[2] PLOS One. "Real-world evaluation of self-collected urine HPV DNA kits in population screening" (Link)
[3] ScienceDirect. Comparison of Urine vs. Swab HPV Test Kits (Link)
[4] WHO, ISO 15189: Medical Laboratories Standards (Link)
[5] FDA EUA database: HPV DNA test devices.
This is the first article
Related PRODUCTS
-
Varicella Zoster Virus PCR Test - Accurate VZV Real Time PCR Detection
NewsJul.26,2025 -
High-Sensitivity Chlamydia DNA PCR Test for Accurate Detection
NewsJul.25,2025 -
Plasmodium Detection via PCR - Fast & Accurate Malaria Diagnostics
NewsJul.24,2025 -
High-Accuracy MTB DNA PCR Test for Rapid TB Detection
NewsJul.23,2025 -
Accurate Norovirus PCR Testing Solutions for Rapid Detection
NewsJul.22,2025





























