About Taizhou Cowingene Biotech Co.,Ltd.
Taizhou Cowingene Biotech Co.,Ltd. stands at the forefront of molecular diagnostics, integrating twenty years of experience in medical testing innovation. Renowned for its quality-driven research and rapid global delivery, Cowingene delivers advanced PCR solutions for clinical and industrial applications.
Website: https://www.cowingene.com
- Phone: +860523-88350768
- Email: info@cowingene.com
- Mobile: 0523-88350768
- Address: NO.28, Xinlin Road, Taizhou city, Jiangsu Province, China
What is Norovirus PCR?
Norovirus PCR refers to the polymerase chain reaction method used to detect the RNA of norovirus, a leading cause of acute gastroenteritis outbreaks globally [CDC]. RT-PCR (reverse transcription PCR) is recognized as the "gold standard" due to its exceptional sensitivity and specificity for norovirus detection from human specimens such as stool and vomitus.
The molecular detection of norovirus has transformed outbreak response, hospital infection control, and food safety monitoring by enabling rapid identification even at low viral loads, with real-time results to support timely decision-making.
Industry Trends in Norovirus PCR
- Rising Global Demand: Since the COVID-19 pandemic, hospital and public health labs have rapidly expanded their molecular testing infrastructure, encouraging greater adoption of reliable norovirus PCR solutions.
- Multiplexing Capability: Many new assays now simultaneously detect norovirus GI and GII genogroups, reducing procedural time and maximizing laboratory throughput [NCBI].
- Automation and Workflow Efficiency: Automated PCR platforms minimize handling error while increasing accuracy, supporting widespread screening during outbreaks, in food production, and cruise ship health management.
- Quality Control & Regulatory Compliance: Validation against international standards (e.g., ISO 15216) is essential, ensuring results are robust and comparable across industries [ISO 15216].
Technical Parameters Comparison for Norovirus PCR
| Assay | Principle | Target | Sensitivity (LOD) | Turnaround time | Sample Types | Molecular Controls |
|---|---|---|---|---|---|---|
| Cowingene Norovirus Detection Kit (NATBox) | Real-time RT-PCR (Fluorogenic probes) | GI, GII norovirus RNA | ≤100 copies/reaction | 55 minutes | Human stool, Vomitus | Positive, Negative, Internal |
| Industry Kit A | Endpoint RT-PCR | GI, GII norovirus RNA | 500 copies/reaction | 90 minutes | Stool | Positive |
| Industry Kit B | qRT-PCR | GII norovirus RNA | 300 copies/reaction | 70 minutes | Stool, Vomitus | Negative, Internal |
| Lab-developed Method | Conventional RT-PCR | GI norovirus RNA | 1,000 copies/reaction | 110 minutes | Stool | None |
Data Visualization: Technology Trends & Kit Performance
Trends in Norovirus PCR Sensitivity (Limit of Detection over Time)
Kit Workflow Time Comparison (Minutes)
Cowingene NATBox: Performance Indicator Distribution
NATBox vs. Competitor Kit - Specification Radar Chart

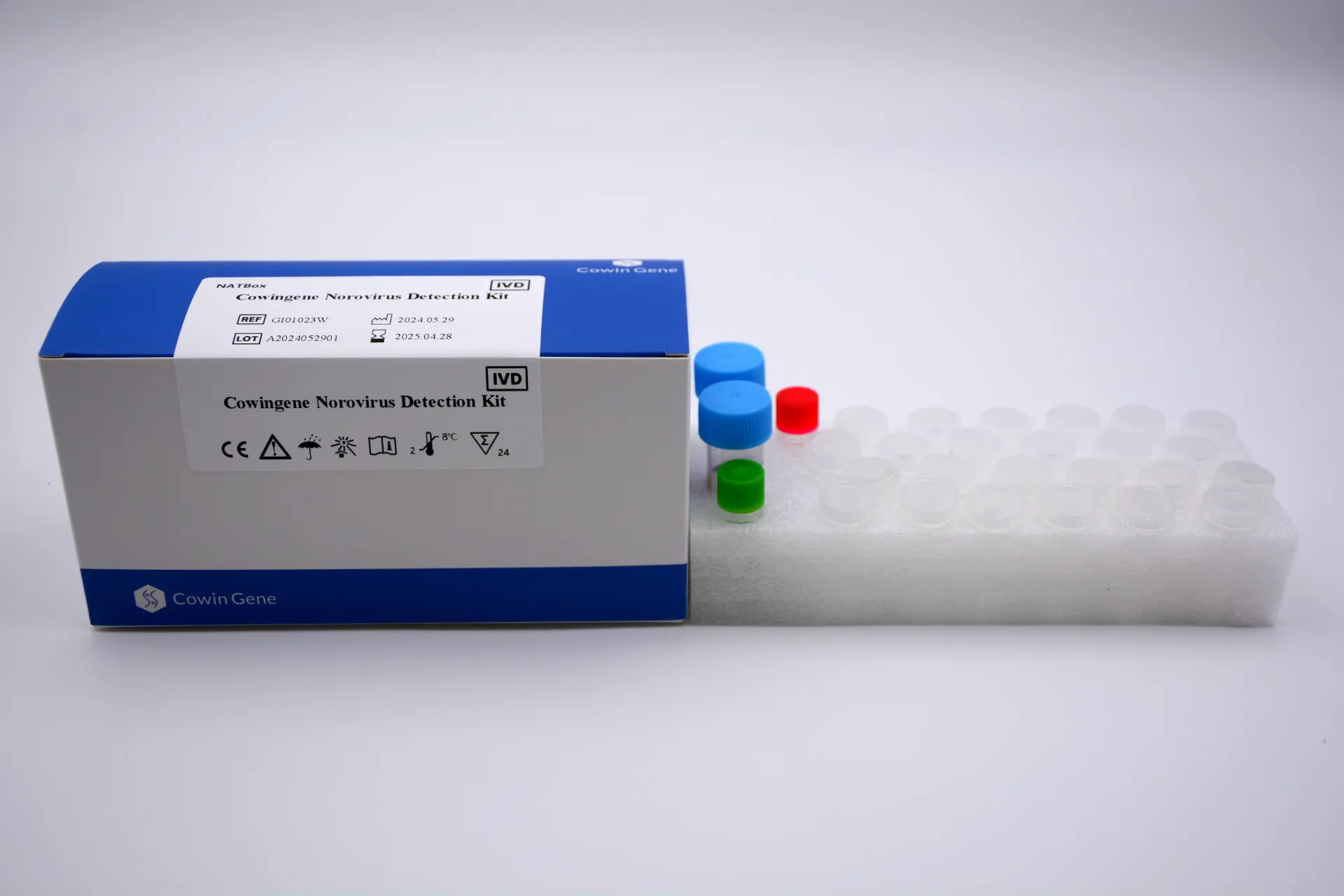
Cowingene Norovirus Detection Kit (NATBox) Overview
Product Name: Cowingene Norovirus Detection Kit (NATBox)
REF: GI01023W
Validated Specimen: Human stool, Vomitus
Analytes: 1 tube; Norovirus RNA
- Ultra-fast results: 55 minutes for up to 96 samples in parallel
- Includes positive, negative, and internal controls for robust validation
- LOD ≤100 copies per reaction enabling early-stage infection detection
- Compatible with major real-time PCR platforms
- Comprehensive GI & GII genotype coverage in one assay
For technical details and documentation, please visit the norovirus pcr product page.
Applications of Norovirus PCR Tests
- Hospital Outbreak Management: Rapid norovirus PCR test is crucial for real-time identification and containment of nosocomial gastroenteritis outbreaks.
- Food Safety & Water Testing: Regulatory agencies use norovirus RT PCR to monitor contamination in food production (especially shellfish), preventing outbreak escalation [Food Safety].
- Cruise Ships & Institutional Health: Periodic large-scale screening helps curb transmission in high-density environments.
- Epidemiological Surveillance: National and international surveillance networks rely on high-sensitivity assays to track genotypes and inform public policy.
- Academic Research: Norovirus molecular testing supports vaccine development, transmission studies, and environmental monitoring [ASM Journal].
EEAT: Scientific Authority & Trusted Validation
Expertise: Cowingene's norovirus solutions are developed by cross-disciplinary teams of molecular diagnosticians and virologists, leveraging extensive field feedback and regulatory guidance. Industry-wide proficiency is demonstrated by the kit's compliance with international standards such as ISO 15216 and continuous validation against updated genotypes.
Authoritativeness: The product is referenced in industry forums and recommended protocols for its reliable detection, aligning with best practices in the diagnosis and management of viral gastrointestinal diseases [PCR Diagnostics Forum].
Trustworthiness: Manufacturing is conducted under certified GMP facilities, with traceable batch controls and publicly available data sheets. Each kit incorporates triplicate controls to guarantee result validity.
Professional FAQ: Norovirus PCR Kit Terminology Explained
Frequently Asked Questions on Norovirus PCR Test Kits
- 1. What is the Analytical Sensitivity (Limit of Detection)?
- The norovirus PCR kit's LOD is ≤100 copies/reaction, ensuring early detection in clinical and environmental samples.
- 2. Which Method is Used for Detection?
- It utilizes real-time RT-PCR, employing fluorogenic probes that allow real-time quantification of norovirus RNA. The norovirus rt pcr approach offers greater sensitivity than conventional PCR.
- 3. What are the Validated Sample Types?
- Human stool and vomitus are validated to guarantee diagnostic accuracy for clinical samples.
- 4. Does the Kit Include Internal Controls?
- Yes, internal, positive, and negative controls are included in each run to monitor extraction efficiency and PCR inhibition.
- 5. What Regulations Does It Meet?
- The kit is tested according to ISO 15216 for food and environmental virology, and meets stringent hospital and public health laboratory requirements.
- 6. Is the Kit Compatible with Automated Platforms?
- Yes, the kit is designed for compatibility with mainstream real-time PCR instruments and most liquid handling systems.
- 7. How Is Result Interpretation Conducted?
- Automated software reads amplification curves, and threshold cycles (Ct-values) correlate to viral load, supporting both qualitative and quantitative analysis.
Future Prospects: Evolution of Norovirus PCR Detection
The field is trending towards digital PCR and increasingly multiplexed panels facilitating wider pathogen coverage and faster, point-of-care deployment. AI-driven interpretation and cloud-based results reporting are enhancing usability and epidemiological impact. Companies like Taizhou Cowingene Biotech Co.,Ltd. will continue to drive these advancements, championing the next generation of norovirus PCR diagnostics.
For technical consultation, demo reservations or partnership inquiries:
- Tel: +860523-88350768 / 0523-88350768
- Email: info@cowingene.com
- Website: www.cowingene.com
- Address: NO.28, Xinlin Road, Taizhou City, Jiangsu Province, China
References & Industry Citations
- CDC Norovirus Laboratory Diagnostics: https://www.cdc.gov/norovirus/lab/diagnostics.html
- Comparison of Norovirus Real-Time RT-PCR Assays: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4302319/
- ISO 15216: https://www.iso.org/standard/65681.html
- FDA Food Safety, Norovirus Testing: https://www.food-safety.com/articles/6485-testing-for-norovirus-in-food-settings
- ASM Journal Norovirus PCR Review: https://journals.asm.org/doi/10.1128/JCM.43.9.4453-4462.2005
- PCR Diagnostics Forum: https://forum.pcr-diagnostics.com/t/norovirus-rt-pcr-kit-review/1223





























