Aug . 11, 2025 13:00 Back to list
Respiratory Pathogen Panel Kits: Rapid & Accurate Testing
Advancing Clinical Diagnostics: The Imperative of Comprehensive Respiratory Pathogen Panels
In the evolving landscape of infectious disease management, the ability to rapidly and accurately identify causative agents of respiratory infections is paramount. A respiratory pathogen panel offers a sophisticated solution, enabling the simultaneous detection of multiple viral and bacterial pathogens from a single clinical sample. This multiplex approach significantly streamlines diagnostic workflows, reduces turnaround times, and facilitates timely clinical decision-making, which is critical for patient outcomes and public health. Beyond individual patient care, these panels play a vital role in epidemiological surveillance, helping to track the spread of respiratory diseases and inform public health interventions, especially in the wake of global health crises. The integration of advanced molecular techniques such as RT-PCR ensures high sensitivity and specificity, making these panels indispensable tools in modern clinical laboratories.
The demand for robust diagnostic tools has never been higher, driven by factors such as emerging infectious diseases, antibiotic resistance concerns, and the need for differentiated diagnoses in patients presenting with similar respiratory symptoms. Comprehensive pathogen identification prevents the overuse of antibiotics, guides appropriate antiviral therapies, and minimizes unnecessary hospitalizations. For B2B stakeholders, investing in cutting-edge respiratory pathogen panel technologies represents a strategic move towards enhancing diagnostic capabilities, improving healthcare efficiency, and ensuring readiness for future health challenges. Understanding the technical nuances and market dynamics of these panels is essential for informed procurement and seamless integration into existing healthcare systems.
Market Dynamics and Emerging Trends in Respiratory Pathogen Testing Kits
The global respiratory pathogen testing kits market has witnessed exponential growth, largely spurred by the recent pandemic and the increasing awareness of co-circulating respiratory viruses. Market reports indicate a compound annual growth rate (CAGR) of over 7% for the coming years, with significant advancements in point-of-care testing (POCT) and multiplex PCR platforms. Key drivers include the rising prevalence of respiratory infections, the growing geriatric population, and continuous technological innovations in molecular diagnostics. Geographically, North America and Europe currently dominate the market due to robust healthcare infrastructure and high adoption of advanced diagnostic solutions, while the Asia-Pacific region is poised for rapid expansion owing to increasing healthcare expenditure and awareness.
Emerging trends point towards higher automation, increased pathogen coverage within a single respiratory pathogen panel, and the development of more rapid, user-friendly systems. The shift from syndromic testing to targeted pathogen identification is also gaining traction, offering more precise patient management and infection control. Laboratories are increasingly seeking solutions that reduce manual intervention, improve throughput, and provide accurate results swiftly, directly impacting patient flow and resource allocation. Manufacturers are responding by integrating AI-powered analytics and cloud connectivity into their platforms, further enhancing diagnostic precision and epidemiological surveillance capabilities for a comprehensive respiratory panel test for various pathogens.
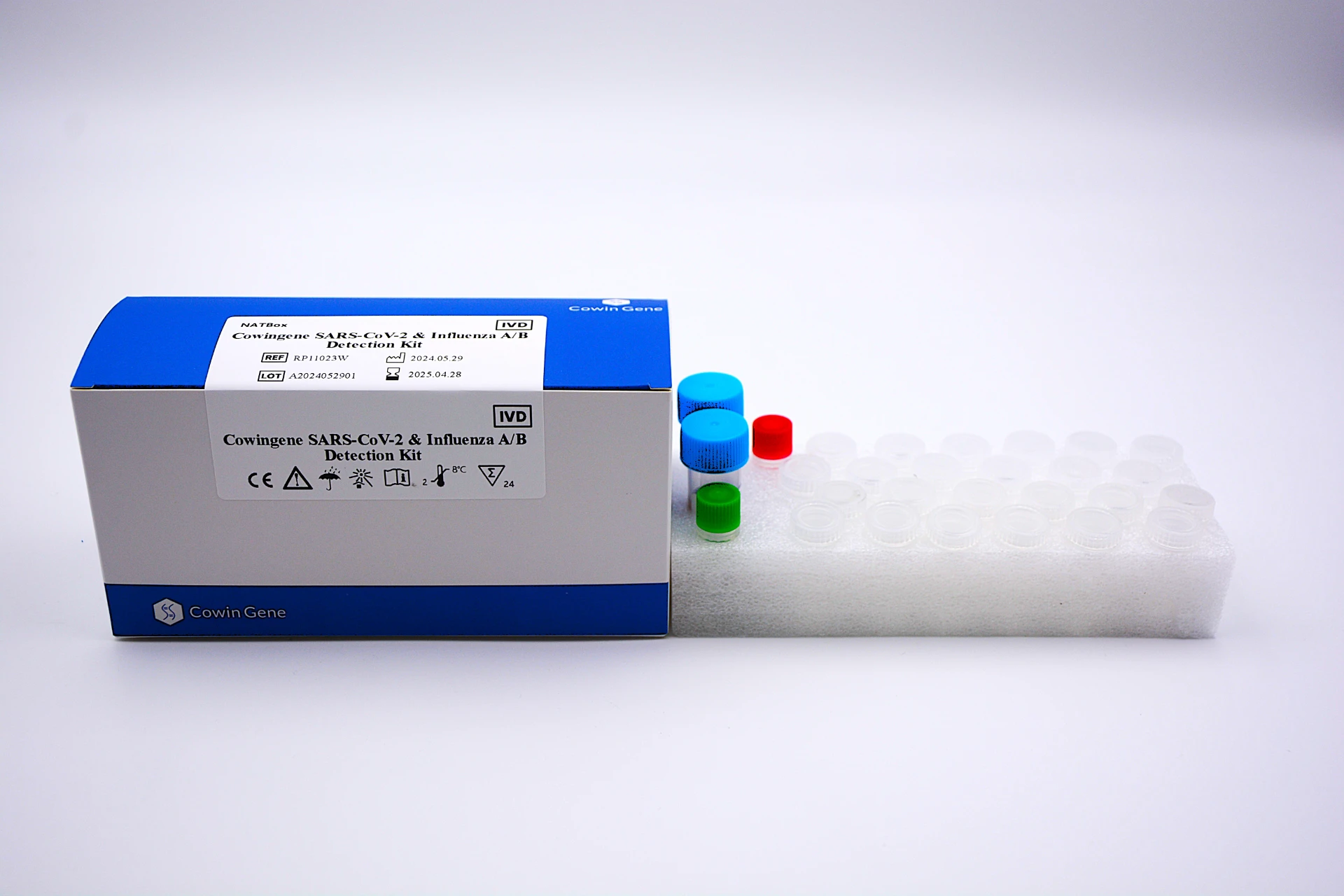
Cowingene's SARS-CoV-2 & Influenza A/B Detection Kit (NATBox): A Technical Overview
Cowingene's SARS-CoV-2 & Influenza A/B Detection Kit (NATBox) exemplifies advanced molecular diagnostic capabilities for comprehensive respiratory testing. This innovative product is designed for the qualitative detection of SARS-CoV-2, Influenza A, and Influenza B viral RNA from respiratory samples, providing a critical respiratory panel test for rapid and accurate diagnosis. Utilizing cutting-edge multiplex real-time RT-PCR technology, the kit offers high sensitivity and specificity, crucial for differentiating between these pathogens that often present with similar clinical symptoms. The NATBox system is engineered for ease of use in diverse clinical laboratory settings, integrating seamlessly into existing workflows and delivering reliable results for effective patient management.
Key technical specifications of the Cowingene NATBox include its multiplex real-time RT-PCR design, allowing simultaneous amplification and detection of multiple targets in a single reaction. The kit's robust performance is validated against a wide range of clinical samples, ensuring reliable results across diverse patient populations and viral loads. Turnaround time for results is typically within 1-2 hours post-nucleic acid extraction, a significant advantage for emergency diagnostics, rapid patient triaging, and effective outbreak management. Its compatibility with standard molecular laboratory equipment further enhances its appeal to B2B clients seeking efficient and scalable diagnostic solutions that meet high regulatory standards.

The Manufacturing & Quality Assurance Process for Diagnostic Panels
The production of a high-quality respiratory pathogen panel like the Cowingene NATBox involves a meticulously controlled multi-stage process, designed to meet stringent international standards such as ISO 13485 for Medical Devices Quality Management Systems. This ensures product reliability, consistency, and efficacy. The initial phase, research and development, focuses on precise oligo (primer and probe) design, careful selection of enzymes, and optimized buffer formulations to maximize sensitivity, specificity, and multiplexing capabilities. Raw materials, including high-purity nucleotides, enzymes, and specialized plastics for reaction tubes and plates, are sourced from certified suppliers and undergo rigorous incoming quality control (IQC) inspections to verify their compliance with strict specifications and ensure downstream performance.
Manufacturing takes place in highly controlled cleanroom environments, minimizing contamination and ensuring aseptic conditions critical for molecular diagnostic reagents. Reagent mixing, precise dispensing, and lyophilization (for stable kits) are performed using automated systems to maintain batch-to-batch consistency. Each batch undergoes comprehensive in-process quality control (IPQC) to monitor key parameters like pH, concentration of components, and absence of non-specific amplification or primer dimers. Finished products are subjected to final quality assurance (FQA) testing, which includes extensive analytical performance verification (e.g., limit of detection, linearity, reproducibility, cross-reactivity) and stability testing (shelf-life validation). This rigorous adherence to cGMP (current Good Manufacturing Practices) principles and ISO standards guarantees the robustness and reliability expected from a premier respiratory pathogen panel in critical healthcare settings.

Comparative Analysis: Cowingene NATBox vs. Standard Panels
When evaluating a respiratory pathogen panel, several key parameters differentiate leading solutions in terms of performance and practical application. The Cowingene SARS-CoV-2 & Influenza A/B Detection Kit (NATBox) offers distinct advantages in precision and efficiency, crucial for high-throughput laboratories and urgent diagnostic needs. Below is a comparative overview highlighting its specific performance metrics against typical market offerings for similar respiratory virus detection.
| Feature | Cowingene NATBox | Standard Respiratory Panel (Typical) |
|---|---|---|
| Pathogens Detected | SARS-CoV-2, Influenza A, Influenza B | Varies (often 3-5 common viruses/bacteria, e.g., RSV, Adenovirus) |
| Assay Type | Multiplex Real-time RT-PCR | Real-time PCR, LAMP, Antigen Test (varies) |
| Sample Type | Nasopharyngeal swabs, Oropharyngeal swabs | Nasopharyngeal swabs, Oropharyngeal swabs, Sputum, BAL |
| Turnaround Time (post-extraction) | ~1-2 hours | ~1.5-4 hours (for PCR-based molecular tests) |
| Sensitivity (e.g., for SARS-CoV-2) | High (>95%, LoD as low as 200 copies/mL) | Generally high for molecular tests (>90%) |
| Specificity (e.g., for SARS-CoV-2) | High (>98%) | Generally high for molecular tests (>95%) |
| Regulatory Status | CE IVD, (may include FDA EUA for specific versions/regions) | Varies by manufacturer and region, often CE IVD / EUA. |
The Cowingene NATBox stands out for its focused detection of critically important respiratory viruses, offering rapid, reliable results that are essential for acute care and public health surveillance. Its high analytical sensitivity and specificity ensure accurate diagnoses, minimizing false negatives and positives, which are crucial for effective disease management and robust infection control protocols in demanding clinical settings.
Application Scenarios and Customer Success Stories
The versatility of Cowingene's respiratory pathogen panel makes it suitable for a wide array of application scenarios across various healthcare settings. In large hospital laboratories, the NATBox facilitates rapid differentiation of viral respiratory infections, allowing for timely isolation of patients and targeted antiviral treatment, thereby preventing nosocomial transmission and optimizing patient flow. Public health laboratories actively utilize the kit for surveillance programs, tracking the prevalence and spread of SARS-CoV-2 and influenza strains, which is vital for informing vaccination strategies and strengthening pandemic preparedness. Additionally, its efficiency and ease of use make it an ideal choice for emergency rooms and urgent care centers where quick diagnostic turnaround is critical for immediate patient triage and management.
One notable example involves a regional hospital network that implemented the Cowingene NATBox during a peak respiratory season. Previously, they struggled with delayed differential diagnoses due to reliance on slower, less comprehensive testing methods that often required multiple assays. After integrating the NATBox, they reported a remarkable 30% reduction in patient turnaround time for respiratory testing results and a significant decrease in empirical antibiotic prescriptions, leading to more appropriate patient care. This led to improved patient flow, optimized resource allocation, and ultimately, better patient outcomes, underscoring the practical benefits of a reliable respiratory panel test for their high-volume demands.
Commitment to Quality, Service, and Trustworthiness
Cowingene is deeply committed to delivering diagnostic solutions that meet the highest standards of quality and reliability. Our unwavering adherence to international certifications such as ISO 13485 underscores our dedication to robust quality management systems throughout product development, manufacturing, and distribution processes. We also proudly hold CE IVD marking, signifying full compliance with European Union health, safety, and environmental protection standards for in vitro diagnostic medical devices. These comprehensive certifications provide our B2B partners with the absolute assurance that our respiratory pathogen panel products are not only technically superior but also globally compliant and consistently trustworthy for critical clinical use.
Our customer support extends well beyond initial product delivery. We offer comprehensive technical assistance, detailed training programs, and proactive troubleshooting to ensure seamless integration and optimal, sustained performance of our kits in your laboratory environment. With a dedicated team of highly skilled experts, we provide prompt responses to all inquiries and offer proactive support for any operational challenges. Our service commitment includes clear and transparent delivery schedules, and while specific warranty terms may vary by product and region, our general policies are designed to ensure product integrity and guaranteed performance. Cowingene aims to build long-term, mutually beneficial partnerships, providing not just cutting-edge products but complete diagnostic solutions tailored to the evolving needs of the dynamic respiratory pathogen testing kits market.
Frequently Asked Questions (FAQ)
Q: What specific pathogens does the Cowingene SARS-CoV-2 & Influenza A/B Detection Kit detect?
A: The kit is specifically designed for the qualitative detection of viral RNA from SARS-CoV-2, Influenza A, and Influenza B, providing a focused respiratory panel test for these epidemiologically significant pathogens.
Q: What is the typical turnaround time for results using the NATBox kit?
A: After nucleic acid extraction from the patient sample, the real-time RT-PCR reaction typically completes within 1-2 hours, allowing for rapid generation of critical diagnostic results essential for timely clinical decisions.
Q: Is the Cowingene NATBox compatible with existing laboratory equipment?
A: Yes, the kit is broadly compatible with common real-time PCR instruments and nucleic acid extraction platforms that are widely utilized in clinical and research laboratories, ensuring seamless integration into your current laboratory setup.
Q: What is the recommended storage and typical shelf-life of the Cowingene SARS-CoV-2 & Influenza A/B Detection Kit?
A: The NATBox kit should typically be stored at -20°C or colder. When stored under these recommended conditions, the typical shelf-life is 12 months from the date of manufacture, ensuring product stability and reliability over time.
Authoritative References
- World Health Organization. (2023). Influenza (Seasonal) - Key facts and figures.
- Centers for Disease Control and Prevention. (2024). Understanding Different Respiratory Viruses and Their Testing.
- European Centre for Disease Prevention and Control. (2023). Surveillance of SARS-CoV-2, influenza and other respiratory viruses in Europe.
- ISO 13485:2016 Medical devices – Quality management systems – Requirements for regulatory purposes.
This is the first article
Related PRODUCTS
-
Mycoplasma Pneumoniae Detection: Accurate PCR Tests
NewsAug.10,2025 -
Reliable Chlamydophila Pneumoniae PCR Test for Accurate Diagnosis
NewsAug.09,2025 -
Accurate Chlamydia Trachomatis Detection | PCR Testing
NewsAug.08,2025 -
Respiratory Panel Lab Tests: Fast & Accurate Pathogen Detection
NewsAug.07,2025 -
Rapid & Accurate MRSA Detection Kit | Fast Results
NewsAug.06,2025